
Botanical extracts and essential oils are prevalent in cosmetics due to the popularity of natural and clean cosmetics. These raw materials are mixtures of numerous chemical compounds, some of which are known to be potentially irritating or allergenic, and others that are normally harmless but can be converted to contact allergens by conditions as simple as exposure to sunlight or oxygen.
Log in to view the full article
Botanical extracts and essential oils are prevalent in cosmetics due to the popularity of natural and clean cosmetics. These raw materials are mixtures of numerous chemical compounds, some of which are known to be potentially irritating or allergenic, and others that are normally harmless but can be converted to contact allergens by conditions as simple as exposure to sunlight or oxygen.
This article explores two types of chemical constituents present in natural extracts and botanicals that, under given conditions, pose allergenic potential to the user: psoralens and terpenes. Examples are provided through the lens of popular ingredients such as bakuchiol, an alternative to retinol, and citrus peels and juices, which are becoming more prevalent as upcycled ingredient sources.
In addition, regulatory considerations for these constituents are described, followed by suggestions for formulators to minimize potential risk.
Bakuchiol, Psoralens and UVA
Bakuchiol has become a highly sought cosmetic ingredient since emerging research continues to position it as a challenger to retinol. This is because bakuchiol targets several cellular pathways in a similar manner, including the modulation of retinoic acid receptor genes, upregulation of collagen and extracellular matrix synthesis.1, 2 Due to this, bakuchiol has been described as a functional analog – a chemical with comparable physical, chemical or pharmacological properties but not necessarily similar in molecular structure to retinol (see Figure 1).
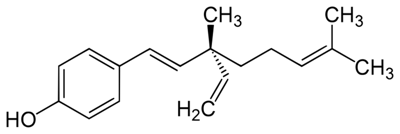 Figure 1. Bakuchiol structure
Figure 1. Bakuchiol structure
In subsequent in vivo evaluations, bakuchiol has demonstrated comparable performance to retinol in the improvement of skin wrinkling and hyperpigmentation but with a lower incidence of irritation than can occur with retinol.3-5 Bakuchiol shows other significant formulation advantages over retinol, namely increased antioxidant capacity, hydrolytic stability and photostability.6,7
The main source of bakuchiol for commercial use is Psoralea corylifolia, also known as the babchi or bakuchi plant, native primarily to China and India. The herb has historically been used in ayurveda and traditional Chinese medicine to treat a variety of skin conditions and other ailments thanks to its antibacterial and anti-inflammatory properties.8 Bakuchiol was first isolated in 1966 from Psoralea corylifolia seeds, where its concentration in the plant is the highest.9, 10
While Psoralea corylifolia is the source of bakuchiol, unpurified extracts and seed oils from the plant can vary widely in composition and quality. The plant and seeds contain a myriad of other phytochemicals including furocoumarins like psoralen and isopsoralen that can be problematic for cosmetic formulation as they may cause serious skin reactions upon exposure to the sun, as described next.11, 12
Psoralens are categorized as furocoumarins, which are a class of compounds characterized structurally by a furan ring fused with a coumarin (see select furocoumarins in Figure 2a-c). Naturally occurring furocoumarins in plants act as insect repellants and pesticides.13 These compounds are known for their potential to cause phototoxic reactions in the skin. They do so by absorbing UVA light in the range of 320 to 400 nm, which forms an excited triplet state that can interact with DNA or undergo reactions with oxygen to produce ROS.14
For example, 5-methoxypsoralen (5-MOP), a psoralen found in citrus peels and juice from grapefruits and limes, can cause photodermatitis – also known as “margarita burn.” Photodermatitis, or photoallergy, is a form of allergic contact dermatitis that appears as a severe sunburn with erythema, blistering and increased pigmentation.15
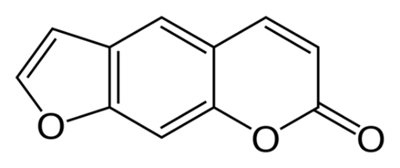 Figure 2a. Structure of psoralen
Figure 2a. Structure of psoralen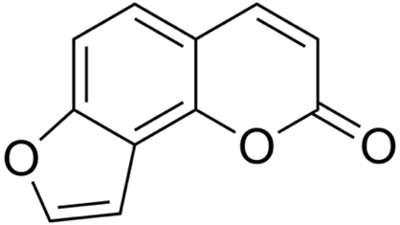 Figure 2 b. Structure of isopsoralen
Figure 2 b. Structure of isopsoralen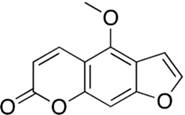 Figure 2c. Structure of 5-methoxypsoralen
Figure 2c. Structure of 5-methoxypsoralen
Psoralens and Cosmetic Safety
As readers know, each cosmetic product should be substantiated for safety before being placed on the market. This appraisal should include a review by an expert safety assessor that involves the evaluation of each ingredient and the full composition – along with relevant testing for irritant and allergenic potential. The safety of ingredients containing psoralens in particular have been a focus in two key markets: the U.S. and Europe.
U.S: In the United States, the presence of psoralens in certain citrus ingredients is addressed by the Cosmetic Ingredient Review (CIR) Expert Panel.16,17 The panel concluded that citrus-derived peel oils, citrus peel-derived ingredients and other citrus fruit-derived ingredients containing psoralens are safe for use in rinse-off and leave-on cosmetic products under given conditions: when formulated to be non-sensitizing and non-irritating; and provided that leave-on products do not contain more than 0.0015% (15 ppm) 5-methoxypsoralen due it its potential for phototoxic reaction in the skin.
Europe: The European Commission’s Scientific Committee on Consumer Products (SCCP) acknowledges that botanical extracts are complex mixtures with many components. Therefore, while the phototoxicity of some furocoumarins like 5-MOP is well-established, the potential impact of numerous other compounds in this class of chemicals has yet to be fully understood. In acknowledging this, SCCP issued an opinion on furocoumarins in cosmetics stating that the total concentration of furocoumarins should not exceed 1 ppm in any finished cosmetic product, irrespective of source.18
Essential Oils, Botanical Extracts, Terpenes and Oxidation
As is well-known, essential oils are fragrant compounds often obtained by the steam distillation of plant parts like flowers, leaves and peels. They find wide use in cosmetics due to their aromatherapeutic properties and as alternatives to synthetic fragrance. Unlike vegetable oils, essential oils are comprised mainly of terpenes and terpenoids.19
Terpenes are the largest group of natural fragrance chemicals and classified by the number of isoprene (C5H8) units present in their structure. The cyclic monoterpene limonene is a component of citrus peel oil as well as eucalyptus essential oils, whereas the acyclic monoterpene linalool is a major component of lavender oil (see Figure 3a-b).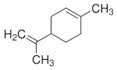 Figure 3a. Structure of limonene
Figure 3a. Structure of limonene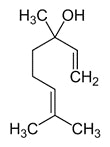 Figure 3b. Structure of linalool
Figure 3b. Structure of linalool
Differences in the chemical composition of oils and extracts can be influenced by plant health, growth stage, habitat and handling of the material itself.20 These constituents can be prone to oxidative damage, leading to undesirable changes in color, odor and viscosity profile and impacting the stability of finished goods.
Exposure to visible and UV light as well as elevated temperatures will accelerate autoxidation21 – and oxidation can change normally innocuous chemicals into allergens. This is the case with two common components of essential oils and fragrances: linalool and limonene.22-24
Terpenes and Cosmetic Safety
In addition to citrus peel oils, flower- and leaf-derived ingredients have also been reviewed by the CIR. The panel concluded that these ingredients are safe for use in rinse-off and leave-on cosmetic products when they are in products formulated to be non-sensitizing and non-irritating. However, the panel expressed concern over the hydroperoxides of limonene and linalool possibly present in these botanical ingredients, noting the industry should use good manufacturing practices to limit impurities.25
As common components of fragrances, limonene and linalool are also within the scope of review by the International Fragrance Association (IFRA). Utilizing the scientific and technical expertise of the industry, IFRA promotes the safe use of fragrance and is responsible for maintaining a Code of Practice26 to which members agree to adhere.
IFRA standards are based on scientific evidence and can prohibit, limit or set purity requirements for specific ingredients.27 The association has issued standards for limonene and linalool and natural products known to be rich in these components, stating these ingredients: “should only be used when the level of peroxides is kept to the lowest practical level, for instance by adding antioxidants at the time of production.”28
Additionally, the Research Institute for Fragrance Materials suggests fragrance ingredients should have a peroxide value (PV) of less than 20 millimoles of peroxides per liter.28 PV measures the degree of oxidation that has already occurred.
Minimizing Risk
There are precautions the cosmetic formulator and ingredient manufacturer can take to minimize the potential for reactions with these chemical entities. In the case of bakuchiol, the formulator should be vigilant of low quality or adulterated materials claiming to be equivalent to purified bakuchiol. There are often higher concentrations of psoralens in low quality extracts.
Bear in mind that in order for an ingredient to display the INCI name Bakuchiol, it should be greater than 80% pure. Other bakuchiol plant extracts would be identified properly as Psoralea corylifolia plant extracts. Still, to protect consumers against injury and comply with regulations, it is imperative to verify the composition and purity of the bakuchiol prior to using it in cosmetic and personal care products.
In the case of terpenes, to minimize their possible oxidation, the handling and storage of botanical ingredients should be specified to minimize exposure to various conditions that could lead to oxidation; e.g., UV light and elevated temperatures.
Conclusion
Botanical extracts are complex mixtures of chemical constituents that while normally safe for use in cosmetics, can undergo changes that result in allergenic potential. Furthermore, multiple botanicals are often combined in cosmetics, increasing the possibility that these compounds could become problematic.
Thorough safety assessments should be performed and special attention should be paid when formulating with these materials. Additionally, proper stability and storage conditions for materials of botanical origin must be prioritized.
References
1. Chaudhuri, R. K.; Bojanowski, K. (2014). Bakuchiol: a retinol‐like functional compound revealed by gene expression profiling and clinically proven to have anti‐aging effects. International journal of cosmetic science. Available at https://pubmed.ncbi.nlm.nih.gov/24471735/
2. Chaudhuri, R. K.; Sivamani, R.; Jagdeo, J.; Elsner, P.; Maibach, H. (2015). Bakuchiol: a retinol-like functional compound, modulating multiple retinol and non-retinol targets. Cosmeceuticals and active cosmetics. Available at https://www.researchgate.net/publication/282896987_Bakuchiol_A_Retinol-Like_Functional_Compound_Modulating_Multiple_Retinol_and_Non-Retinol_Targets
3. Greenzaid, J.; Friedman, A.; Sodha, P. (2022). The Use of Bakuchiol in Dermatology: A Review of In Vitro and In Vivo Evidence. Journal of Drugs in Dermatology: JDD. Available at https://pubmed.ncbi.nlm.nih.gov/35674758/
4. Dhaliwal, S.; Rybak, I.; Ellis, S.; Notay, M.; Trivedi, M.; Burney, W.; Vaughn, A.; Nguyen, M.; Reiter, P.; Bosanac, S. (2019). Prospective, randomized, double‐blind assessment of topical bakuchiol and retinol for facial photoageing. British Journal of Dermatology. Available at https://pubmed.ncbi.nlm.nih.gov/29947134/
5. Draelos, Z. D.; Gunt, H.; Zeichner, J.; Levy, S. (2020). Clinical Evaluation of a Nature-Based Bakuchiol Anti-Aging Moisturizer for Sensitive Skin. Journal of Drugs in Dermatology: JDD. Available at https://pubmed.ncbi.nlm.nih.gov/33346506/
6. Bluemke, A.; Ring, A. P.; Immeyer, J.; Hoff, A.; Eisenberg, T.; Gerwat, W.; Meyer, F.; Breitkreutz, S.; Klinger, L. M.; Brandner, J. M. (2022). Multidirectional activity of bakuchiol against cellular mechanisms of facial ageing‐Experimental evidence for a holistic treatment approach. International Journal of Cosmetic Science. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9328396/
7. Chaudhuri, R.; Ou, B. (2015). Bakuchiol to stabilize retinol and polyunsaturated lipids. Cosmetics & Toiletries. Available at https://www.cosmeticsandtoiletries.com/cosmetic-ingredients/article/21835331/bakuchiol-to-stabilize-retinol-and-polyunsaturated-lipids#:~:text=Bakuchiol%2C%20a%20meroterpene%20of%20plant,imparts%20in%20different%20oxidative%20environments.
8. Alam, F.; Khan, G. N.; Asad, M. H. H. B. (2018). Psoralea corylifolia L: Ethnobotanical, biological, and chemical aspects: A review. Phytotherapy Research. Available at https://pubmed.ncbi.nlm.nih.gov/29243333/
9.Mehta, G.; Nayak, U.; Dev, S. (1973). Meroterpenoids—I: Psoralea corylifolia Linn.—1. bakuchiol, a novel monoterpene phenol. Tetrahedron. Available at https://www.semanticscholar.org/paper/Meroterpenoids%E2%80%94I-%3A-Psoralea-corylifolia-Linn.%E2%80%941.-a-Mehta-Nayak/dc5bece6b97d538ee0770e6c13164497dfab82cc
10. Mehta, G.; Nayak, U. R.; Dev, S. (1966). Bakuchiol, a novel monoterpenoid. Tetrahedron Letters. Available at https://www.sciencedirect.com/science/article/abs/pii/S0040403900700785
11. Faulkner, J.; Uthayakumar, A. K.; Atkins, J. (2021). Babchi oil-induced phytophotodermatitis mimicking burn injury. JPRAS open. Available at https://pubmed.ncbi.nlm.nih.gov/33299922/#:~:text=Due%20to%20its%20psoralen%20containing,the%20UK%2C%20presenting%20as%20sunburn.
12. Brown, A. M.; Ahmed, A. (2022). High Frequency of Photosensitizers in Products Marketed Online for Vitiligo. Dermatitis. Available at https://www.liebertpub.com/doi/abs/10.1097/DER.0000000000000849?journalCode=der
13. Klocke, J. A.; Balandrin, M. F.; Barnby, M. A.; Yamasaki, R. B. (1989). Limonoids, phenolics, and furanocoumarins as insect antifeedants, repellents, and growth inhibitory compounds. ACS Publications. Available at https://pubs.acs.org/doi/10.1021/bk-1989-0387.ch010
14. Hermanson, G. (2013). Bioconjugate Techniques. Available at https://www.sciencedirect.com/book/9780123822390/bioconjugate-techniques
15. Lovell, C.; Paulsen, E.; Lepoittevin, J.-P. (2020). Adverse skin reactions to plants and plant products. Contact Dermatitis. Available at https://link.springer.com/referenceworkentry/10.1007/978-3-030-36335-2_88
16. Burnett, C. L.; Fiume, M. M.; Bergfeld, W. F.; Belsito, D. V.; Hill, R. A.; Klaassen, C. D.; Liebler, D. C.; Marks Jr, J. G.; Shank, R. C.; Slaga, T. J. (2019). Safety assessment of citrus-derived peel oils as used in cosmetics. International journal of toxicology. Available at https://pubmed.ncbi.nlm.nih.gov/31522650/
17. Burnett, C. L.; Bergfeld, W. F.; Belsito, D. V.; Hill, R. A.; Klaassen, C. D.; Liebler, D. C.; Marks Jr, J. G.; Shank, R. C.; Slaga, T. J.; Snyder, P. W. (2021). Safety Assessment of Citrus Fruit-Derived Ingredients as Used in Cosmetics. International journal of toxicology. Available at https://pubmed.ncbi.nlm.nih.gov/34410830/#:~:text=The%20Panel%20concluded%20that%20these,citrus%20fruit%3B%20cosmetics%3B%20safety.
18. Products, (2005). S. C. o. C. SCCP/0942/05 Opinion on Furocoumarins in Cosmetic Products. European Commission Health and Consumer Protection Directorate General. Available at https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_036.pdf
19. O'lenick, A. J.; Jr.; et al. (2008). Oils of nature. Sternberg & Associates. Available at https://www.steinbergandassociates.com/images/pubs/Oils-of-Nature.pdf
20. Schmidt, E. (2010). Handbook of essential oils: science, technology, and applications. Handbook of essential oils science, technology, and applications. Boca Raton: Taylor and Francis Group, LLC. Available at https://www.routledge.com/Handbook-of-Essential-Oils-Science-Technology-and-Applications/Baser-Buchbauer/p/book/9780367504021
21. Turek, C.; Stintzing, F. C. (2013). Stability of essential oils: a review. Comprehensive reviews in food science and food safety. Available at https://ift.onlinelibrary.wiley.com/doi/10.1111/1541-4337.12006
22. Karlberg, A.-T.; Shao, L.; Nilsson, U.; Gäfvert, E.; Nilsson, J. (1994). Hydroperoxides in oxidized d-limonene identified as potent contact allergens. Available at https://www.sciencedirect.com/science/article/pii/S2352512620302757#:~:text=Limonene%20on%20its%20own%20has%20low%20sensitization%20potential.&text=However%2C%20upon%20exposure%20to%20air,to%20cause%20allergic%20contact%20dermatitis.
23. Bråred Christensson, J.; Karlberg, A. T.; Andersen, K. E.; Bruze, M.; Johansen, J. D. Garcia‐Bravo, B.; Giménez Arnau, A.; Goh, C. L.; Nixon, R.; White, I. R. (2016). Oxidized limonene and oxidized linalool–concomitant contact allergy to common fragrance terpenes. Contact Dermatitis. Available at https://pubmed.ncbi.nlm.nih.gov/26918793/
24. Christensson, J. B.; Matura, M.; Gruvberger, B.; Bruze, M.; Karlberg, A. T. (2010). Linalool–a significant contact sensitizer after air exposure. Contact dermatitis. Available at https://pubmed.ncbi.nlm.nih.gov/20136877/
25. Burnett, C. L.; Bergfeld, W. F.; Belsito, D. V.; Hill, R. A.; Klaassen, C. D.; Liebler, D. C.; Marks Jr, J. G.; Shank, R. C.; Slaga, T. J.; Snyder, P. W. (2021). Safety Assessment of Citrus Flower-and Leaf-Derived Ingredients as Used in Cosmetics. International journal of toxicology. Available at https://pubmed.ncbi.nlm.nih.gov/34747255/
26. IFRA (2021) IFRA Code of Practice. IFRA. Available at https://ifrafragrance.org/about-ifra/ifra-code-of-practice 27. Pybus, D. H.; Sell, C. S. (1999). The chemistry of fragrances; Royal Society of Chemistry. Available at https://vinumvine.files.wordpress.com/2010/01/10010011.pdf
28. Api, A.; Boyd, J.; Renskers, K. (2015). Peroxide levels along the fragrance value chain comply with IFRA standards. Flavour and Fragrance Journal. Available at https://onlinelibrary.wiley.com/doi/10.1002/ffj.3257










