
Globally, according to Fortune Business Insights, the tattoo market is projected to grow from $2.04 billion in 2023 to $3.93 billion by 2030 – a CAGR of 9.87%.1 In Brazil alone, according to the Brazilian Support Service for Micro and Small Businesses (SEBRAE), from 2016 to 2017, the tattoo market was unhindered by the country’s economic crisis and grew at rate of nearly 20% per year.1 In this context, this increase can be correlated with higher consumer demand for tattoos.
Log in to view the full article
Globally, according to Fortune Business Insights, the tattoo market is projected to grow from $2.04 billion in 2023 to $3.93 billion by 2030 – a CAGR of 9.87%.1 In Brazil alone, according to the Brazilian Support Service for Micro and Small Businesses (SEBRAE), from 2016 to 2017, the tattoo market was unhindered by the country’s economic crisis and grew at rate of nearly 20% per year.1 In this context, this increase can be correlated with higher consumer demand for tattoos.
As is well-known, tattooing involves the process of repeatedly inserting pigment into the dermis through a machine that can pierce the skin three thousand times per minute to create a permanent design. Tattoos designs can hold personal meaning, including serving aesthetic and therapeutic purposes such as eyebrow filling and camouflaging areas with pigment (vitiligo) or hair loss (alopecia) concerns, among others.2
However, tattooing can bring about complications such as allergies, inflammation, infections and neoplasms.2 The insertion of pigment into the dermis causes damage, generating an immune response from the body. Defense cells recognize the pigment as an antigen and initiate an inflammatory response, possibly leading to necrosis.
Considering the level of skin damage and immune response, it is clear that tattooed skin could benefit from extra care and specific elements to provide such;3, 4 for example, vitamins with antioxidant and moisturizing properties, such as D-panthenol and vitamin C. D-panthenol is known for its emollient, repairing, moisturizing and anti-inflammatory properties, which can accelerate cell regeneration.5, 6 Also, fat-soluble vitamin C (ascorbyl tetraisopalmitate), which has excellent antioxidant activity, can promote protection from UVA/UVB exposure and help to maintain the color of the tattoo.7-9
Despite the increase in tattooing, few studies address the use of products for tattooed skin, making the development of stable cosmetic products with proven clinical efficacy an interesting proposition for tattooed skin care. The present study therefore aimed to develop a cosmetic formulation for tattooed skin incorporating D-panthenol and ascorbyl tetraisopalmitate. Stability, spreadability and sensory evaluations were carried out in addition to clinical studies of the skin’s microrelief and moisture content.
Stability and Sensory Analysis
Test formula preparation: Two test formulations were developed with or without (placebo) both D-panthenol and ascorbyl tetraisopalmitate, according to the specifications of each active. Sensory properties and possible interactions with the vehicle composition were considerations. The base formula (F1) was a gel-cream comprising: sclerotium gum, glycerin, cetearyl alcohol (and) ceteareth-20, caprylic/capric triglyceride, benzyl alcohol (and) xylitol (and) caprylic acid, and disodium EDTA; the test formula (F2) additionally included D-panthenol (1%) and ascorbyl tetraisopalmitate (3%).
Stability and formula characterization: pH measurements, centrifugation and the analysis of organoleptic characteristics were carried out every seven days for 28 days. The pH value was verifieda using an aqueous dispersion of each formulation at 10% (w/w).10, 11 Centrifugationb was carried out in triplicate at 3,000 rpm for 30 min after 24 hr and 28 days using 3 g of each formulation. In addition, samples were visually observed for changes in color, odor and homogeneity at 24 hr and weekly for 28 days at RT (≈ 25°C), 37°C and 45°C.12
Rheological behavior analysis: The rheological behavior was determined using a digital rheometerc. Formulations were stored at RT (≈ 25°C) and subjected to thermal stress at 37°C and 45°C. For the analysis, aliquots of 0.50 ± 0.01 g were collected at time intervals of seven days for 28 days.13, 14
The rotation speed was progressively increased from 0 to 40 rpm with 20 sec between each speed, forming an ascending curve with 10 points composed by the axes "shear rate" versus "shear stress." A descending curve was then generated with an inverse decrease in velocity.11
Texture and spreadability analysis: Texture and spreadability profile analyses were performed in quintuplicate after 24 hr and 28 days using a texturometerd. This method is based on inserting an analytical probe into the sample with previously defined speed and depth. Two different probes were used to test for spreadabilitye and texture profilef.11, 15, 16
Sensory analysis: Sensorial analysis was performed in an untrained panel of 31 healthy participants between the ages of 18 and 29 with skin phototypes of II to IV. They applied the test products F1 and F2 and responded to questionnaires asking whether they agreed, partially agreed or disagreed about the product’s spreadability, smoothness, hydration sensation and stickiness.11, 17 For this test, two 20-cm² circles were delimited in the anterior region of the forearms. F1 and F2 were each applied to one of the circles in a standardized manner; i.e., spread by the participants 20 times within the delimited area. Immediately after product application, the participants responded to the questionnaire in terms of product spreadability and smoothness; five minutes after application, participants rated stickiness and hydration.
Short-term Clinical Efficacy Study
After approval by the ethics committee, 16 participants were recruited through the laboratory's clinical trial participant bank. The inclusion criteria were: individuals aged between 18 and 39 years, with skin phototypes of II and III, female or male, healthy and with a tattoo on their arm. Subjects who agreed to participate signed an informed consent form.
Regions of the arm or forearm where the tattoo was present were divided into two, to which each of formulation F1 or F2 was applied. Measurements were taken before (baseline, T0) and two hours after (T2h) product application. Skin without tattooing was used as a control. Stratum corneum (SC) water content, water distribution and the skin microrelief were then evaluated. All measurements were performed in triplicate.
SC water content: SC water content was measured using a corneometerg based on the principle of electrical capacitance. The results were provided in arbitrary units (AU), where 1 AU corresponds to 0.2 - 0.9 mg of water per gram of SC.18
Determination of skin microrelief: Optical profilometry was used to capture the cutaneous microrelief. Here, a specialized UV-A light video camera with high resolutionh scanned the skin to provide qualitative and quantitative information about its surface under physiological conditions.20, 21
Water distribution: A specialized devicej used for moisture mapping evaluated hydration distribution in the skin. This measurement is based on the GrayIndex or dark pixel index,19 wherein a sensor measures the penetration of an electromagnetic field into skin, and conductive material such as water reflects the signal, resulting in darker pixels. Non-conductive material permits the signal travel farther, resulting in a pixel that is lighter by 255 grey levels.
Statistical analysis and presentation of results: The experimental data was submitted to statistical analysisk and represented in tables and figures with discussion based on data found in the literature.
Results and Discussion: Stability and Sensory Analysis
Stability and formula characterization: The formulations did not show phase separation 24 hours and 28 days after preparation, appearing homogeneous and therefore considered stable in this test. Furthermore, changes in color, odor, homogeneity and phase separation were not observed during the study period when kept at room temperature or subjected to thermal stress. The pH values remained in the range of 4.5 to 5.5 at all times and temperatures studied, thus the formulas were considered compatible with the pH of the skin.22, 23
Rheological behavior: Formulations F1 and F2 showed a non-Newtonian rheological behavior of the thixotropic pseudoplastic type, with a flow index of < 1 and viscosity recovery after shear reduction.11
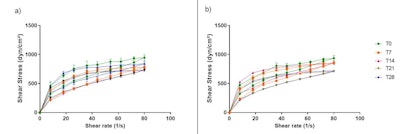 Figure 1. Rheograms of formulations F1 (a) and F2 (b), from T0 to T28, stored at RT
Figure 1. Rheograms of formulations F1 (a) and F2 (b), from T0 to T28, stored at RT
Per the rheograms, the rheological behavior of the formulations was stable throughout the 28 days under the three temperature conditions tested; no peaks or changes in the rheograms were observed during the analysis period (see Figure 1). Furthermore, the addition of active substances did not alter the rheological profile of the formulations.
Thixotropy indicates the time the material takes to reorganize itself and return to the initial structure after the shear is removed.24 The hysteresis area, resulting from the thixotropy phenomenon, is calculated between the ascending and descending curves. Thus, F1 presented a higher hysteresis area value when compared to F2, showing that the addition of active substances decreased the time that the structure of the formulation took to reorganize itself; however, this difference was not significant (p > 0.05) at T0 or T28.
Texture and spreadability: In addition, sensory properties and clinical performance must be balanced to develop effective and consumer-acceptable cosmetic formulations. These properties depend on the texture profile, whose analysis is critical for the planning and development of cosmetic formulations.11, 14, 25, 26
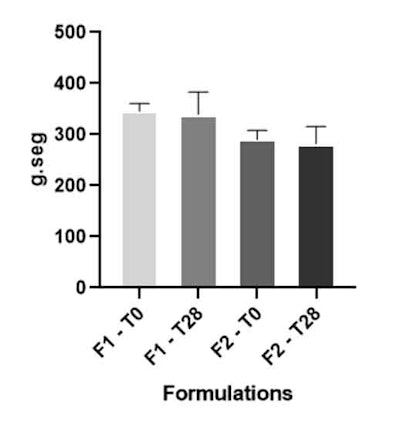 Figure 2. Work of shear parameter of formulations F1 and F2 at T0 and T28
Figure 2. Work of shear parameter of formulations F1 and F2 at T0 and T28
The work of shear parameter is inversely related to the spreadability of the formulation over the skin. Therefore, the lower the work of shear, the lower the force required to spread the formulation on the skin and the better the spreadability.11, 14, 26 Test formula F2 presented less shear work (see Figure 2), which corroborated the data obtained in the sensory perception analysis, where most participants considered it to have the best spreadability. Firmness, consistency and cohesiveness parameters showed no significant difference (p < 0.05) after 28 days of study.
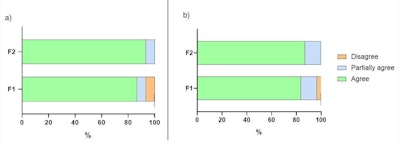 Figure 3. Sensory evaluation of spreadability (A) and smoothness (B) parameters immediately after application of formulations F1 and F2.
Figure 3. Sensory evaluation of spreadability (A) and smoothness (B) parameters immediately after application of formulations F1 and F2.
Sensory analysis: The values obtained from the sensory questionnaires regarding panelists’ application of F1 and F2 were plotted according to the pre-established scale of agree/partially agree/disagree. From this, it was possible to identify some sensory differences between the formulas. Immediately after application, in terms of smoothness, panelists agreed F2 was just slightly smoother than F1. However, in analyzing spreadability, for F1, 13% of the participants only partially agreed or disagreed that the formulation “spread well on the skin'' (see Figure 3).
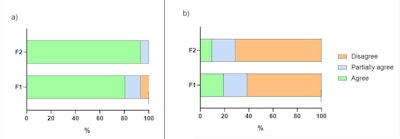 Figure 4. Sensory evaluation of hydration (A) and stickiness (B) parameters after five minutes of application of formulations F1 and F2.
Figure 4. Sensory evaluation of hydration (A) and stickiness (B) parameters after five minutes of application of formulations F1 and F2.
Five minutes after application, the hydration and stickiness of formulations were evaluated, which provided more noticeable differences. Here, it was agreed the F2 formulation provided the best hydration sensation and least stickiness (see Figure 4). When asked about their preference between the formulations, 70.97% of participants chose the F2 formulation, reinforcing the positive influence of the additional actives.
Results and Discussion: Clinical Study
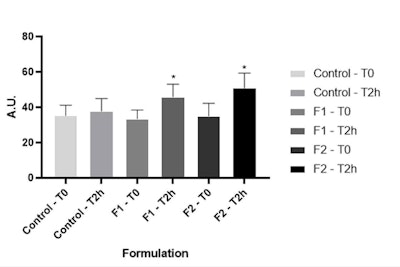 Figure 5. Stratum corneum water content at time zero and after two hours of formulations F1 and F2 application. *Significant difference compared to baseline values (p < 0.05).
Figure 5. Stratum corneum water content at time zero and after two hours of formulations F1 and F2 application. *Significant difference compared to baseline values (p < 0.05).
SC water content: Regarding the clinical study, two hours after both formulas were applied, a significant increase in the stratum corneum water content was observed compared to their respective baseline values (see Figure 5). This indicates the vehicle formulation (F1) also had a moisturizing action. This is probably due to both the addition of glycerin, which can act in the hydration and restoration of the SC,27 as well as the fatty composition of the base used. However, even if not significant (p > 0.05), F2 showed a higher increase in the SC water content than F1, suggesting the presence of the actives had an impact.
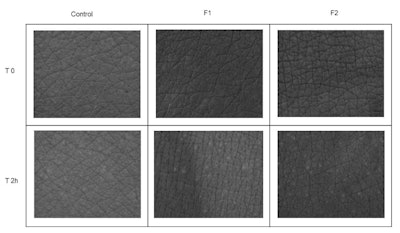 Figure 6. Comparison of skin microrelief at time zero (T0) and after two hours (T2) of application of formulations F1 and F2. The skin without the tattoo was used as a control.
Figure 6. Comparison of skin microrelief at time zero (T0) and after two hours (T2) of application of formulations F1 and F2. The skin without the tattoo was used as a control.
Determination of skin microrelief: The high-resolution imagesh showed an improvement in cutaneous microrelief two hours of application of the F2 formulation, with uniformity in the reduction of the depth of skin creases and homogeneity of the skin surface (see Figure 6). F1 imparted similar improvements but to a lesser extent, indicating the additional actives helped to potentiate skin hydration in the short term.
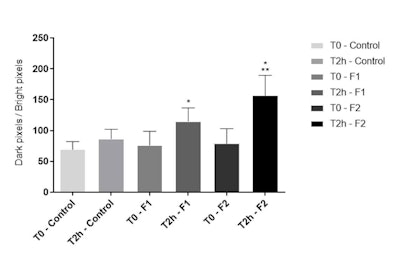 Figure 7. Skin hydration distribution; GrayIndex parameter at T0 and T2h after application of F1 and F2
Figure 7. Skin hydration distribution; GrayIndex parameter at T0 and T2h after application of F1 and F2
Water distribution: Both formulations F1 and F2 significantly increased the GrayIndex parameter (p < 0.05) compared with baseline values. However, at T2h, F2 significantly (p < 0.05) increased skin hydration distribution, compared with F1 (see Figure 7). This shows that the added actives improved the hydration of tattooed skin, supporting and corroborating the previously discussed results in terms of increased water content in the SC.
Conclusion
The present work aimed to develop a cosmetic formula for tattooed skin incorporating D-panthenol and ascorbyl tetraisopalmitate. Bother base and test formulas, without and with actives, respectively, remained stable throughout the 28 days of stability testing and demonstrated pseudoplastic rheological behavior compatible with the proposed application – for tattooed skin. In terms of texture, the test formulation including actives required less work of shear than the base formula, as shown by spreadability testing. Furthermore, no variations in texture or spreadability profiles were observed after 28 days.
The results of the short-term clinical efficacy study showed that after two hours of application, the test formulation incorporating the actives had a more pronounced moisturizing effect than the base formulation. This included a significant increase in water distribution in the skin and the SC water content, as well as the improvement of the cutaneous microrelief. Thus, the test formulation incorporating D-panthenol and ascorbyl tetraisopalmitate proved to be stable, with smooth and stable characteristics, and effective for improving hydration in tattooed skin.
Acknowledgments: The authors wish to thank Fapesp – São Paulo Research Foundation (grant number: 2021/10186-0), NEATEC - Nucleus of Advanced Studies in Cosmetic Technology of the School of Pharmaceutical Sciences of Ribeirão Preto – USP, and the study participants.
a Digimed DM20 pH meter
b Excelsa Baby II centrifuge
c DV-III digital rheometer, cone and plate type with shear rate control, Brookfield
d TA XT Plus Texturometer with Exponent software, Extralab Brasil
e TTC Spreadability Rig (HDP/SR)
f Back Extrusion rig (A/BE)
g Corneometer CM 825
h Visioscan VC 98 and j Moisture MAP MM 100, Courage & Khazaka
k Prism 8 and Origin 8 software
References
1. Market Research Report (2022). The global tattoo market is projected to grow from $2.04 billion in 2023 to $3.93 billion by 2030, at a CAGR of 9.87% during the forecast period. Fortune Business Insights. Available at https://www.fortunebusinessinsights.com/tattoo-market-104434
2. Temiz, S. A.; Özlü, E. (2021). Medical Complications of Tattoos. Journal of the Turkish Academy of Dermatology. Available at https://jtad.org/archives/archive-detail/article-preview/medical-complications-of-tattoos/46856
3. Kluger, N.; Koljonen, V. (2012). tattoos, inks, and cancer. The lancet oncology. Available at https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045%2811%2970340-0/fulltext#:~:text=Several%20studies%20have%20shed%20light,cases%20of%20basal%2Dcell%20carcinoma.
4. Farley, C. L.; Van Hoover, C.; Rademeyer, C. A. (2019). Women and tattoos: Fashion, meaning, and implications for health. Journal of midwifery e women's health. Available at https://pubmed.ncbi.nlm.nih.gov/30806488/
5. Aresenie, L.V.; Lacatusu, I.; Oprea, O.; Bordei, N.; Bacalum, M.; Badea, N. (2020). Azelaic acid-willow bark extract-panthenol–Loaded lipid nanocarriers improve the hydration effect and antioxidant action of cosmetic formulations. Industrial Crops and Products. Available at https://www.sciencedirect.com/science/article/abs/pii/S0926669020305744
6. Hroboňová, K.; Lomenova, A. (2020). Determination of panthenol enantiomers in cosmetic preparations using an achiral‐chiral–coupled column HPLC system. Chirality. Available at https://pubmed.ncbi.nlm.nih.gov/31788853/
7. Maia Campos, P. M. B. G.; Gianeti, M.; Camargo JR, F.; Gaspar, L. (2012). Application of tetra-isopalmitoyl ascorbic acid in cosmetic formulations: Stability studies and in vivo efficacy. European Journal of Pharmaceutics and Biopharmaceutics. Available at https://pubmed.ncbi.nlm.nih.gov/22974986/
8. Jeon, J.; Kim, H.; Kim, M.; Oh, M.; Hong, S.; Yoon, M.; Abd El-Aty, A. (2016). Simultaneous detection of glabridin,(−)-α-bisabolol, and ascorbyl tetraisopalmitate in whitening cosmetic creams using HPLC-PAD. Chromatographia. Available at https://link.springer.com/article/10.1007/s10337-016-3104-2
9. Narda, M.; Brown, A.; Muscatelli-Groux, B.; Grimaud, J.; Granger, C. (2020). Epidermal and Dermal Hallmarks of Photoaging are Prevented by Treatment with Night Serum Containing Melatonin, Bakuchiol, and Ascorbyl Tetraisopalmitate: In Vitro and Ex Vivo Studies. Dermatology and Therapy. Available at https://pubmed.ncbi.nlm.nih.gov/31900804/
10. Davis, H. (1977). Analysis of creams and lotion. In: Newburguer's manual of cosmetic analysis. Washington: Association of Official Analytical Chemists, Senzel, AJ. Available at https://books.google.com/books/about/Newburger_s_Manual_of_Cosmetic_Analysis.html?id=aJy4PAAACAAJ
11. Calixto, L.; Maia Campos, P. M. B. G. M. 2017). Physical Mechanical characterization of cosmetic formulations and correlation between instrumental measurements and sensorial properties. International journal of cosmetic science. Available at https://pubmed.ncbi.nlm.nih.gov/28555924/
12. Infante, V. H. P.; Maia Campos, P. M. B. G. M.; Calixto, L.; Darvin, M.; Kröger, M.; Schanzer, S.; Meinke, M. (2021). Influence of physical–mechanical properties on SPF in sunscreen formulations on ex vivo and in vivo skin. International Journal of Pharmaceutics. Available at https://pubmed.ncbi.nlm.nih.gov/33549814/
13. Gaspar, L.; Maia Campos, P. M. B. G. (2003). Rheological behavior and the SPF of sunscreens. Inter. J. Pharma. Available at https://pubmed.ncbi.nlm.nih.gov/12480271/
14. Calixto, L.; Infante, V. H. P.; Maia Campos, P. M. B. G. (2018). Design and Characterization of Topical Formulations: Correlations Between Instrumental and Sensorial Measurements. AAPS PharmSciTech. Available at https://pubmed.ncbi.nlm.nih.gov/29464591/
15. Behera, B.; Singh, V.; Kulanthaivel, S.; Bhattacharya, M.; Paramanik, K.; Banerjee, I. (2015). Physical and mechanical properties of sunflower oil and synthetic polymers based bigels for the delivery of nitroimidazole antibiotic—a therapeutic approach for controlled drug delivery. Eur Polym J. Available at https://www.sciencedirect.com/science/article/abs/pii/S0014305715000300
16. Gilbert, L.; Savary, G.; Grisel, M.; Picard, C. (2013). Predicting sensory texture properties of cosmetic emulsions by physical measurements. Chemom Intell Lab Syst. Available at https://www.sciencedirect.com/science/article/abs/pii/S016974391300035X
17. Marcon, A., Wagemaker, T.; e Campos, P. (2014). Rheology, clinical efficacy and sensorial of a silicone-based formulation containing pearl extract. Biomedical and Biopharmaceutical Research. Available at https://www.researchgate.net/publication/292125377_Rheology_clinical_efficacy_and_sensorial_of_a_silicone-based_formulation_containing_pearl_extract
18. Dal’Belo, S.; Gaspar, L.; Maia Campos, P. M. B.G. (2006). Moisturizing effect of cosmetic formulations containing Aloe vera extract in different concentrations assessed by skin bioengineering techniques. Skin Research and Technology. Available at https://pubmed.ncbi.nlm.nih.gov/17026654/
19. Leite Gabarra, M.; Maia Campos, P. M. B. G. M. (2020). Correlations between sebaceous gland activity and porphyrins in oily skin and hair and immediate effects of dermocosmetic formulations. Journal of Cosmetic Dermatology. Available at https://pubmed.ncbi.nlm.nih.gov/32185849/
20. Levy, J.; Trelles, M.; Servant, J.; Agopian, L. (2004). Non-ablative skin remodeling: an 8-month clinical and 3D in vivo profilometric study with an 810 nm diode laser. J.Cosmet. Laser.Ther. Available at https://pubmed.ncbi.nlm.nih.gov/15370407/
21. Melo, M.; Maia Campos, P.M.B.G. (2019). Application of biophysical and skin imaging techniques to evaluate the film‐forming effect of cosmetic formulations. International journal of cosmetic science. Available at https://pubmed.ncbi.nlm.nih.gov/31469171/
22. Maia Campos, P. M. B. G.; Gianeti, M.; Camargo JR, F.; Gaspar, L. (2012). Application of tetra-isopalmitoyl ascorbic acid in cosmetic formulations: Stability studies and in vivo efficacy. European journal of pharmaceutics and biopharmaceutics. Available at https://pubmed.ncbi.nlm.nih.gov/22974986/
23. Andrade, J.; Wagemaker, T.; Mercurio, D.; Maia Campos, P. M. B. G. (2018). Benefits of a dermocosmetic formulation with vitamins B3 and a B6 derivative combined with zinc-PCA for mild inflammatory acne and acne-prone skin. Biomed Biopharm Res. Available at https://www.researchgate.net/publication/330031190_Benefits_of_a_dermocosmetic_formulation_with_vitamins_B3_and_a_B6_derivative_combined_with_zinc-PCA_for_mild_inflammatory_acne_and_acne-prone_skin_Beneficios_de_uma_formulacao_dermocosmetica_com_vitam
24. Chorilli, M.; Campos, G.; Bolfarini, P. (2009). Desenvolvimento e estudo da estabilidade físico-química de emulsões múltiplas A/O/AEO/A/O acrescidas de filtros químicos e manteiga de karité. Latin American Journal of Pharmacy. Available at https://www.researchgate.net/publication/268286332_Desenvolvimento_e_Estudo_da_Estabilidade_Fisico-Quimica_de_Emulsoes_Multiplas_AOA_E_OAO_Acrescidas_de_Filtros_Quimicos_e_Manteiga_de_Karite
25. Gore, E.; Picard, C.; Savary, G. (2018). Spreading behavior of cosmetic emulsions: Impact of the oil phase. Biotribology. Available at https://www.sciencedirect.com/science/article/abs/pii/S2352573818300143
26. Terescenco, D.; Picard, C.; Clemenceau, F.; Grisel, M.; Savary, G. (2018). Influence of the emollient structure on the properties of cosmetic emulsion containing lamellar liquid crystals. Colloids and Surfaces A: Physicochemical and Engineering Aspects. Available at https://www.sciencedirect.com/science/article/abs/pii/S0927775717307720
27. Stettler, H.; Kurka, P.; Lunau, N.; Manger, C.; Böhling, A.; Bielfeldt, S.; Lenz, H. (2017). A new topical panthenol-containing emollient: Results from two randomized controlled studies assessing its skin moisturization and barrier restoration potential, and the effect on skin microflora. Journal of Dermatological Treatment. Available at https://pubmed.ncbi.nlm.nih.gov/27425824/










