Female hair loss has gained increasing attention in recent years, impacting not only aesthetic appearance but also potentially the psychological well-being and overall quality of life for women.1, 35 Consequently, researching and developing products for preventing female hair loss has become a high priority area. This was the focus of the present research, wherein kudzu isoflavones were explored for the potential to signal the hair growth cycle in dermal papilla.
Log in to view the full article
Female hair loss has gained increasing attention in recent years, impacting not only aesthetic appearance but also potentially the psychological well-being and overall quality of life for women.1, 35 Consequently, researching and developing products for preventing female hair loss has become a high priority area. This was the focus of the present research, wherein kudzu isoflavones were explored for the potential to signal the hair growth cycle in dermal papilla.
Hormone levels in women change throughout physiological cycles and with age. Particularly, as women age, estrogen levels gradually decrease2 while the relative proportion of androgens, such as testosterone, may increase. This can lead to the gradual shrinking of hair follicles, a shortened growth cycle and ultimately, result in hair thinning and loss.3 Additionally, environmental4 and genetic factors5 play an important role in female hair loss.
Dermal papilla cells, situated at the base of hair follicles, are mesenchymal cells that serve as signaling centers for epithelial-mesenchymal interactions throughout the hair growth cycle.6, 7 These can be considered the command center of hair follicles. Dermal papilla cells induce the proliferation and differentiation of follicle-related cells by secreting various signaling growth factors (e.g., VEGF, KGF, IGF, HGF, FGF, TGF-β2, etc.),8 thereby regulating the hair growth cycle and governing the development of follicles and hair growth.9
Bone Morphogenetic Protein-2(BMP-2), belonging to the TGF-β superfamily of proteins,10 is one of the growth signals secreted by dermal papilla cells11 that acts as a signaling protein to promote the transition of hair from the resting phase to a new growth phase.12 As such, this regulatory factor was identified as a biomarker for exploration.
Although the relationship between BMP-2 signaling in the hair cycle has mostly been studied in mice, Midorikawa, et al., conducted a DNA macroarray analysis on DPCs from androgenetic alopecia (AGA) patients. They found that compared with DPCs from unaffected skin, the BMP-2 gene expression was reduced in AGA patients. Additionally, in vitro cell experiments demonstrated that BMP-2 stimulated the growth of hair follicle epithelial cells or induced keratin synthesis.13 These findings suggest that BMP-2 could act as a promoter in hair cycling.
Furthermore, Mundy, et al., conducted two independent clinical studies based on the following evidences: 1) proteasome inhibitors stimulate skeletal formation in mice through a BMP-2-mediated mechanism;14 and 2) a recent report in a patient treated with recombinant BMP-2 for a fractured tibia showed evidence of increased hair growth.15 The results by Mundy, et al., confirmed that topical application of proteasome inhibitors to the scalp could induce the anagen phase and stimulate hair growth for men with androgenetic alopecia.16 In other words, promoters of BMP-2 expression could induce the anagen phase and stimulate hair growth.
Also, research indicates that female pattern hair loss and male balding share a final common pathway of follicular regression.17 Zhou, et al.,18 discovered, through a murine model, that estrogen could upregulate the expression of BMP-2 mRNA in mesenchymal stem cells (MSCs). They also observed a baseline reduction in BMP-2 mRNA levels in MSCs when the ovaries of the mice were surgically removed.19, 20
Based on this evidence, the present authors hypothesized that estrogen in women could promote the expression of BMP-2 in DPCs, while a decline in estrogen levels could lead to a decrease in overall scalp BMP-2 levels, ultimately causing female hair loss. Consequently, a Pueraria lobata (kudzu) extract, known for its phytoestrogen content, was chosen as a potential BMP-2 promoter and investigated for its effects on the expression of BMP-2 in DPCs.
Sometimes referred to as phytoestrogens, isoflavones have estrogen-like effects.21, 22 Kudzu root, a traditional Chinese medicine used in various prescriptions,23-25 is rich in isoflavones26 that have garnered significant interest due to their antioxidant properties,27 neuroprotective effects28 and the ability to reduce blood alcohol levels.29
The present work describes in vitro studies to determine whether kudzu extract could promote BMP-2 expression in dermal papilla cells. Following this, a hair tonic was developed featuring kudzu extract and other key ingredients and clinically tested to examine the formula’s potential to mitigate female hair loss.
Materials and Methods
Kudzu isoflavones and BMP-2 synthesis: Human dermal papilla cells (ACI3047) were seeded into a 96-well plate and pre-cultured for one day under conditions of 37°C and 5.0% CO2. Kudzu isoflavonesa were then added to the wells at 0% isoflavones (control; i.e., butylene glycol vehicle only), 0.5% and 1.0%, and the cells were further cultured for an additional two days under the same conditions. Subsequently, the supernatant from each culture was collected and the BMP-2 content in the culture supernatant was determined using an ELISA kitb.
Anti-hair loss effects of test tonic: The test hair loss prevention tonicc comprised: alcohol, water (aqua), butylene glycol, the Pueraria lobata root extract (0.5%)a, Arctium lappa root extract, Panax ginseng root extract, Saccharomyces/Coix lacryma-jobi ma-yuen seed ferment filtrate, Stephania cephalantha root extract, dipotassium glycyrrhizate and Mentha viridis (spearmint) leaf oil.
Clinical test panelists and protocol: Forty eligible Chinese female participants ages 31 to 59 experiencing hair loss and hair thinning were randomly divided into two groups for the test active or control treatment (see Table 1). Prior to testing, participants completed the informed consent forms; testing was carried out between September and November 2023.
For initial testing, upon arrival to the test facilityd, participants acclimated for 30 min in a lab maintained at 21 ± 2°C and 50 ± 10% RH. A lab technician carried out a 60-stroke comb test from root to tip and participants with more than 10 hairs lost were included as official participants; this hair loss data was recorded.
Photographs were then taken by lab technicians who marked a fixed area of at least 1.5 cm × 1.5 cm on the participant's scalp, on the temporal side of the crown, ensuring hair was trimmed to no longer than 1 mm at each visit. Photos were captured using an electronic dermatoscopee and tool to analyze local hair densityf; a dermatologist also evaluated hair density based on the photographs.
The technician then explained the product application method to participants: both groups applied 5 mL of either the hair loss prevention tonic or control (water) once daily for 28 days. Technicians distributed samples and supervised the initial use. Upon each subsequent visit, the same protocol was followed, participants’ usage logs were checked and test samples were weighed to ensure compliance. Clinical hair loss counts and local hair density (image analysis) photographs were taken at baseline (Day 0), Day 14 and Day 28 of product use.
Results: BMP-2 Synthesis In vitro
The effects of kudzu isoflavones on BMP-2 synthesis in vitro, as depicted in Figure 1 (shown below), demonstrated that 0.5% and 1.0% concentrations significantly enhanced the synthesis of BMP-2 in human dermal papilla cells. The promotional effects on synthesis were 22× and 23.67× that of the control, respectively.
Results: Hair Loss Prevention In vivo
Table 2, below, illustrates the clinical results in terms of hair loss counts. Here, the mean represents the average hair loss count; MD0 represents the within-group difference between D14 and D0, and D28 and D0 – with the difference indicating the average decrease or increase in hair loss.
In participants who used the test tonic, the mean hair loss count was significantly reduced by 36.28% (p < 0.05) after 14 days, and after 28 days, by 45.73% (p < 0.01). These results showed statistically significant differences (p < 0.001) compared with the control group, which showed a non-significant increase in hair loss over the same period. Statistical analyses were performed using the paired t-test.
Table 3, below, illustrates the results of local hair density measurements. Again, the mean represents the average local hair density; MD0 represents the within-group difference between D14 and D0, and D28 and D0 – with the difference indicating the average increase or decrease in local hair density.
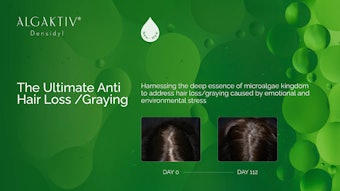
In the group of participants using the test tonic, the mean local hair density significantly increased by 29.55% (p < 0.01) after 14 days, and 32.60% (p < 0.001) after 28 days. These results demonstrated significant differences (p < 0.001) compared with the control group; in the control group, an insignificant but slight increase in local hair density was observed. Statistical analyses were performed using the paired t-test. A representative example of the effects after 28 days of using the hair loss prevention tonic is illustrated in Figure 2, shown below.
Discussion
It is well-recognized that factors influencing hair loss are complex, including:
- hormone levels,
- environmental factors (seasonal hair loss),
- genetic predisposition and
- overall scalp conditions.
Therefore, an effective anti-hair loss cosmetic should combine multiple active ingredients targeting multiple factors, particularly for scalp health improvement. As such, the intention of this work was not to prove the clinical anti-hair loss efficacy of kudzu isoflavones per se, but rather the efficacy of the tonic containing the kudzu isoflavones.
The described tonic included the hair cycle-related BMP-2 promoter (kudzu isoflavones), in addition to dipotassium glycyrrhizate, for anti-inflammatory effects to improve the scalp environment; Panax ginseng root extract to enhance blood circulation; a Stephania cephalantha root extract to revitalize DPCs;30, 31 Arctium lappa root extract to promote type XVII collagen synthesis in follicular cells;32 and other plant extracts for sebum and antioxidant purposes, to enhance scalp barrier function, etc.33
Notably, in the clinical trial, hormonal levels were not standardized and living environments among participants were not controlled. As such, variations in lifestyle and habits could have led to physiological and psychological changes that, to some degree, may have influenced the outcomes of the study.
This trial did not seek to eliminate these uncertainties, but rather to reflect the dynamics of real-life situations. In addition, since the described work focused on a specialized cosmetic for hair loss prevention, rather than a therapeutic drug – which would require a much longer testing period, a one-month testing period was deemed reasonable.
Conclusion
In the present work, kudzu isoflavones showed a significant improvement in BMP-2 expression in dermal papilla cells in vitro. Additionally, a hair loss prevention tonic featuring kudzu isoflavones significantly reduced hair loss and improved local hair density in human clinical trials. These results suggest a new product solution for female hair loss concerns.
Footnotes
a Technoble Concentrated Kudzu Isoflavones (INCI: Water (Aqua) (and) Butylene Glycol (and) Pueraria Lobata Root Extract)
b DBP200, R&D Systems, USA
c OR Medicated Hair Tonic, O and R Co., Ltd.
d Studies were carried out by SGS CSTC Standards Technical Services Co., Ltd., Xiamen Branch
e Dermoscan
f Trichoscan
References
1. Cash, T.F., Price, V.H. and Savin, R.C. (1993). Psychological effects of androgenetic alopecia on women: Comparisons with balding men and with female control subjects. J Amer Acad Derm, 29(4), 568-575; https://doi.org/10.1016/0190-9622(93)70223-G
2. Lamberts, S.W.J., van den Beld, A.W. and van der Lely, A.-J. (1997). The endocrinology of aging. Science, 278(5337), 419-424; https://doi.org/10.1126/science.278.5337.419
3. Ramos, P.M. and Miot, H.A. (2015). Female pattern hair loss: A clinical and pathophysiological review. Anais Brasileiros de Dermatologia, 90(4), 529-543; https://doi.org/10.1590/abd1806-4841.20153370
4. Ramos, P.M., Brianezi, G., Martins, A.C.P., da Silva, M.G., Marques, M.E.A. and Miot, H.A. (2017). Aryl hydrocarbon receptor overexpression in miniaturized follicles in female pattern hair loss. Anais Brasileiros de Dermatologia, 92(3), 430-431; https://doi.org/10.1590/abd1806-4841.20175150
5. Redler, S., Messenger, A.G. and Betz, R.C. (2017). Genetics and other factors in the aetiology of female pattern hair loss. Experimental Dermatology, 26(6), 510-517; https://doi.org/10.1111/exd.13373
6. Alonso, L. and Fuchs, E. (2006). The hair cycle. J Cell Science, 119(3), 391-393; https://doi.org/10.1242/jcs.02793
7. Schmidt-Ullrich, R. and Paus, R. (2005). Molecular principles of hair follicle induction and morphogenesis. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology, 27(3), 247-261; https://doi.org/10.1002/bies.20184
8. Wang, J.M. and Zhang, J.T. (2012). Progress in relevant growth factors promoting the growth of hair follicle. American Journal of Animal and Veterinary Sciences, 7(2) 104-111; https://doi.org/10.3844/ajavsp.2012.104.111
9. Madaan, A., Verma, R., Singh, A.T. and Jaggi, M. (2018). Review of hair follicle dermal papilla cells as in vitro screening model for hair growth. Intl J Cos Sci, 40(5), 429-450; https://doi.org/10.1111/ics.12489
10. Wang, R.N., Green, J., ... Shi, L.L., et al. (2014). Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes & Diseases, 1(1), 87-105; https://doi.org/10.1016/j.gendis.2014.07.005
11. Tomochika, M., Sumie, R., Miyano, R., Mochizuki, Y., Inoue, H. and Kajikawa, A. (2022). Possible cellular communication with human follicle dermal papilla cells via secretions from human hair follicle keratinocytes. J St. Marianna University, 13(2), 87-93; https://doi.org/10.17264/stmarieng.13.87
12. Ohnemus, U., Uenalan, M., Inzunza, J., Gustafsson, J.-A. and Paus, R. (2006). The hair follicle as an estrogen target and source. Endocrine Reviews, 27(6), 677-706; https://doi.org/10.1210/er.2006-0020
13. Midorikawa, T., Chikazawa, T., Yoshino, T., Takada, K. and Arase, S. (2004). Different gene expression profile observed in dermal papilla cells related to androgenic alopecia by DNA macroarray analysis. J Dermatol Sci, 36, 25-32; doi:10.1016/j.jdermsci.2004.05.001.
14. Garrett, I.R., Chen, D., ...Hu, S., et al. (2003). Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest, 111, 1771-1782; doi:10.1172/JCI200316198.
15. Leslie, K.S., Shah, S.N., Darrah, C., Cooper, A., Valentin-Opran, A., Patel, A.D. and Donel, S.T. (2006). Alopecia universalis treated with bone morphogenetic protein? Br J Dermatol, 154, 190-191; doi:10.1111/j.1365-2133.2005.07011.x.
16. Mundy, G., Gutierrez, G., Garrett, R., Gallwitz, W., Rossini, G., Christiansen, C. and Langenberg, A. (2007). Proteasome inhibitors stimulate both bone formation and hair growth by similar mechanisms. Annals of the New York Academy of Sciences, 1117, 298-301; doi:10.1196/annals.1402.077.
17. Redler, S., Messenger, A.G. and Betz, R.C. (2017). Genetics and other factors in the aetiology of female pattern hair loss. Exp Dermatol, 26, 510-517; doi:10.1111/exd.13373.
18. Zhou, S., Turgeman, G., Harris, S.E., Leitman, D.C., Komm, B.S., Bodine, P.V.N. and Gazit, D. (2003). Estrogens activate bone morphogenetic protein-2 gene transcription in mouse mesenchymal stem cells. Molecular Endocrinology, 17(1), 56-66; https://doi.org/10.1210/me.2002-0210
19. Turgeman, G., Zilberman, ... Gazit, D., et al. (2002). Systemically administered rhBMP-2 promotes MSC activity and reverses bone and cartilage loss in osteopenic mice. J Cellular Biochem, 86(3) 461-474; https://doi.org/10.1002/jcb.10231
20. Zhou, S., Zilberman, Y., Wassermann, K., Bain, S.D., Sadovsky, Y. and Gazit, D. (2001). Estrogen modulates estrogen receptor alpha and beta expression, osteogenic activity and apoptosis in mesenchymal stem cells (MSCs) of osteoporotic mice. Journal of Cellular Biochemistry, suppl 36, 144-155; https://doi.org/10.1002/jcb.1096
21. Lee, Y.S., Park, J.S., Cho, S.D., Son, J.K., Cherdshewasart, W. and Kang, K.S. (2002). Requirement of metabolic activation for estrogenic activity of Pueraria mirifica. Journal of Veterinary Science, 3(4), 273-277.
22. Malaivijitnond, S., Chansri, K., Kijkuokul, P., Urasopon, N. and Cherdshewasart, W. (2006). Using vaginal cytology to assess the estrogenic activity of phytoestrogen-rich herb. Journal of Ethnopharmacology, 107(3) 354-360; https://doi.org/10.1016/j.jep.2006.03.026
23. Guerra, M.C., Speroni, E., ...Paolini, M., et al. (2000). Comparison between Chinese medical herb Pueraria lobata crude extract and its main isoflavone puerarin antioxidant properties and effects on rat liver CYP-catalyzed drug metabolism. Life Sciences, 67(24), 2997-3006; https://doi.org/10.1016/s0024-3205(00)00885-7
24. Tham, D.M., Gardner, C.D. and Haskell, W.L. (1998). Clinical review 97: Potential health benefits of dietary phytoestrogens: A review of the clinical, epidemiological and mechanistic evidence. The Journal of Clinical Endocrinology and Metabolism, 83(7), 2223-2235; https://doi.org/10.1210/jcem.83.7.4752
25. Yu, B.-S., Yan, X.-P., Zhen, G.-B. and Rao, Y.-P. (2002). RP-HPLC determination of puerarin in Chinese traditional medicinal preparations containing pueraria. Journal of Pharmaceutical and Biomedical Analysis, 30(3) 843-849; https://doi.org/10.1016/s0731-7085(02)00326-6
26. Kirakosyan, A., Kaufman, P.B., Warber, S., Bolling, S., Chang, S.C. and Duke, J.A. (2003). Quantification of major isoflavonoids and l-canavanine in several organs of kudzu vine (Pueraria montana) and in starch samples derived from kudzu roots. Plant Science, 164(5), 883-888; https://doi.org/10.1016/S0168-9452(03)00077-3
27. Kang, K.A., Chae, S., Koh, Y.S., Kim, J.S., Lee, J.-H., You, H.J. and Hyun, J.W. (2005). Protective effect of puerariae radix on oxidative stress induced by hydrogen peroxide and streptozotocin. Biological & Pharmaceutical Bulletin, 28(7), 1154-1160; https://doi.org/10.1248/bpb.28.1154
28. Wang, Z.-Y., Wei, X.-B., Chen, L., Liu, P., Wang, L.-X., Zhang, B., Sun, X. and Zhang, X.-M. (2007). Neuroprotective effects of hydroxyethylpuerarin against focal cerebral ischemia-reperfusion in rats. The Chinese Journal of Physiology, 50(5), 211-216.
29. Lin, R.C., Guthrie, S., Xie, C.Y., Mai, K., Lee, D.Y., Lumeng, L. and Li, T.K. (1996). Isoflavonoid compounds extracted from Pueraria lobata suppress alcohol preference in a pharmacogenetic rat model of alcoholism. Alcoholism, Clinical and Experimental Research, 20(4), 659-663; https://doi.org/10.1111/j.1530-0277.1996.tb01668.x
30. Inui, S. and Itami, S. (2013). Induction of insulin-like growth factor-I by cepharanthine from dermal papilla cells: A novel potential pathway for hair growth stimulation. The Journal of Dermatology, 40, 1054-1055; doi:10.1111/1346-8138.12269.
31. Inui, S.,Tohyama, C. and Itami, S. (2016). Acceleration of hair growth rate by topical liposomal cepharanthine in male androgenetic alopecia. In Proceedings of the Hair Therapy & Transplantation, vol. 06; available at https://www.longdom.org/open-access-pdfs/acceleration-of-hair-growth-rate-by-topical-liposomal-cepharanthine-in-maleandrogenetic-alopecia-2167-0951-1000145.pdf
32.Tanimura, S., Tadokoro, Y., ... Sawamura, D., et al. (2011). Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell, 8, 177-187; doi:10.1016/j.stem.2010.11.029.
33. Wölfle, U., Haarhaus, B., Seiwerth, J., Cawelius, A., Schwabe, K., Quirin, K.-W. and Schempp, C.M. (2017). The herbal bitter drug Gentiana lutea modulates lipid synthesis in human keratinocytes in vitro and in vivo. Int J Mol Sci, 18, 1814; doi:10.3390/ijms18081814.
34. Shimizu, G., Wedy, G., Schaefer, L., Ramos, P. and Miot, H. (2018). Translation into Portuguese language (Brazil), transcultural adaptation and validation of the quality of life questionnaire in female pattern hair loss (WAA-QoL-BP). Anais Brasileiros de Dermatologia, 93, 701-706; https://doi.org/10.1590/abd1806-4841.20187452