
The following article was provided by Chemlinked and compiles information on seven major regions that have regulations for fragrance allergen labeling, in addition to their specific labeling requirements. Update: On July 26, 2023, the European Commission added 45 new entries to the Annex. These are added in Section 4 of this report (EU).
Log in to view the full article
The following article was provided by Chemlinked and compiles information on seven major regions that have regulations for fragrance allergen labeling, in addition to their specific labeling requirements. Update: On July 26, 2023, the European Commission added 45 new entries to the Annex. These are added in Section 4 of this report (EU).
1. Chinese Mainland
Adult Cosmetics
In 2018, the National Committee on the Assessment of the Protected Traditional Chinese Medicinal Products (Center for Food Evaluation, State Administration for Market Regulation), commented on the issue of cosmetic fragrance allergen labeling on its official website.
Cosmetic products often contain fragrances or vegetable oils in which allergens may exist. In some countries/regions, such as the European Union (EU), manufacturers are required to label allergens above a certain concentration on the product label to warn consumers. If such products are sold in the Chinese market, the enterprise must include the warning "This product/ ** ingredient contains [allergen]" in the "precautions" section on the Chinese label. However, such ingredients are not allowed to be listed in the "full ingredients" section, unless they are added separately to the formula.
Related: 11 Changes to SCCS Guides for Cosmetics Safety Testing — Plus a Note on 'Nano'
Children Cosmetics
Technical Guidelines for Children’s Cosmetics (Draft for Comments) released on April 11, 2022, encourage the avoidance of fragrance and flavors in children's cosmetics, particularly those that may contain any of the 26 fragrance allergens listed in Table 1.
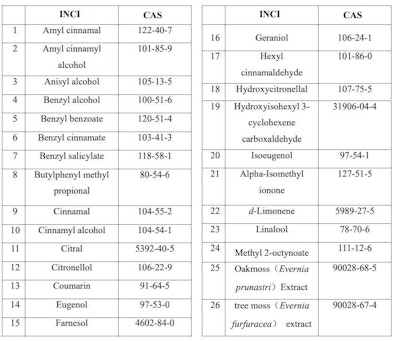 Table 1. Fragrance and Flavor Allergens to Avoid for Children's Cosmetics in China
Table 1. Fragrance and Flavor Allergens to Avoid for Children's Cosmetics in China
If fragrance or flavors containing these 26 allergens are used:
- A full safety assessment must be conducted;
- For leave-on products containing fragrance allergens exceeding 0.001%, or rinse-off products with a concentration of fragrance allergens exceeding 0.01%, the name of the specific fragrance allergens should be labeled, guided by "contains allergens."
This draft requirement for children's cosmetics is significant, as it marks the first time that China has proposed the labeling of the 26 fragrance allergens. This shows that the Chinese regulatory authorities are prioritizing the safety supervision for children's cosmetics.
2. South Korea
Enterprises in South Korea are required to label 25 fragrance allergens on the “full ingredients” section on cosmetic packaging, but only when they exceed certain concentrations. Specifically, rinse-off products require labeling when fragrance allergens exceed 0.01%, while leave-on products require labeling when fragrance allergens exceed 0.001%. The 25 allergens that must be labeled and their CAS numbers are listed in Table 2.
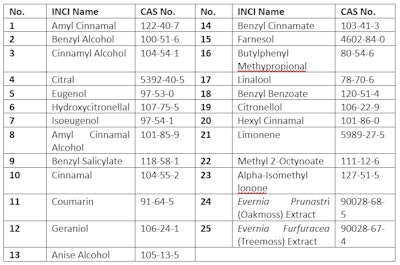 Table 2. South Korea – 25 Fragrance Allergens that Must Be Labeled
Table 2. South Korea – 25 Fragrance Allergens that Must Be Labeled
3. India
India requires companies to indicate the presence of 26 fragrance allergens in the list of ingredients when concentrations exceed 0.01% in rinse-off products and 0.001% in leave-on products. The 26 allergens that must be labeled and their CAS numbers are listed in Table 3.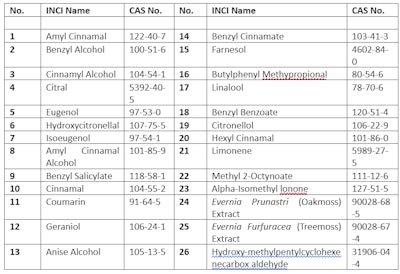 Table 3. India – 26 Fragrance Allergens that Must Be Labeled
Table 3. India – 26 Fragrance Allergens that Must Be Labeled
4. EU
The EU requires companies to indicate the presence of 24 fragrance allergens in the list of ingredients when concentrations exceed 0.01% in rinse-off products and 0.001% in leave-on products, whether or not they are added directly as an ingredient or are present as a component of a fragrance ingredient. The 24 allergens that must be labeled and their CAS numbers are listed in Table 4.
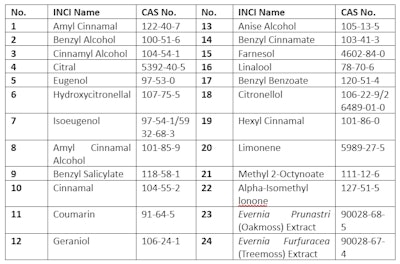 Table 4. EU - 24 Fragrance Allergens that Must Be Labeled
Table 4. EU - 24 Fragrance Allergens that Must Be Labeled
Expanding the Scope of Fragrance Allergens
The European Commission released a draft notification (G/TBT/N/EU/924) on Sept. 15, 2022, proposing revisions to the labeling of fragrance allergens in cosmetics under Regulation (EC) No. 1223/2009.
The notification mandates the labeling of 56 newly identified fragrance allergens when their concentration exceeds 0.001% in leave-on products or 0.01% in rinse-off products. For a complete list of these allergens, see the European Commission Annex.Update: July 26, 2023
On July 26, 2023, the European Commission (EC) published a new amendment, EU 2023/1545, to EU Cosmetics Regulation 1223/2009, adding 45 entries to the Annex including allergens such as vanillin, terpineol, menthol, Mentha piperita oil and Lippia citriodora absolute, per SGS Digicomply. In total, 80 ingredients and ingredient groups are recognized as allergenic and must be disclosed on cosmetic product labels. See the EU document for the full list of additions.
Furthermore, 17 entries were aligned with the common names of the substances. Other fragrance compounds are also limited or banned, such as Lilial, Atranol and Chloratranol, SGS Digicomply reported.
All the ingredients must be listed on the product container or packaging, in addition to the term parfum or aroma when present above the following levels:
- 0.01% in a rinse-off cosmetic (e.g., soap, shower gel, shampoo) and
- 0.001% in a leave-on cosmetic (e.g. cream, lotion, tonic).
Adjustments to product formulations and packaging will be required to ensure that all products on the market are in compliance, SGS Digicomply emphasized. Considering the number of changes, a longer transition period was provided by the Commission: non-compliant cosmetic products can be placed on the market until July 31, 2026, and made available on the market until July 31, 2028.
5. UK
The UK's labeling requirements for fragrance allergens are consistent with those of the EU (see Table 4). Companies must indicate the presence of 24 fragrance allergens in the list of ingredients when concentrations exceed 0.01% in rinse-off products and 0.001% in leave-on products, regardless of whether they are added directly as an ingredient or are present as a component of a fragrance ingredient.
6. New Zealand
New Zealand requires companies to indicate the presence of 26 fragrance allergens in the list of ingredients when concentrations exceed 0.01% in rinse-off products and 0.001% in leave-on products, whether or not they are added directly as an ingredient or are present as a component of a fragrance ingredient. The 26 allergens that must be labeled and their CAS numbers are listed in Table 5.
 Table 5. New Zealand – 26 Fragrance Allergens that Must Be Labeled
Table 5. New Zealand – 26 Fragrance Allergens that Must Be Labeled
7. The United Arab Emirates (UAE)
Product ingredients must be listed on the container or on the product itself if no container is used. Allergens must be included in the ingredients list on the container label. If a perfume contains an aromatic allergenic substance at a concentration above 0.001%, it must be listed among the substances that compose the product on the container's packaging; however, the UAE did not give a specific list of fragrance allergens.
Legislation in Progress
United States
Currently, cosmetics in the United States are not required to be labeled with fragrance allergens. However, the Modernization of Cosmetics Regulation Act of 2022 (MoCRA), passed on Dec. 29, 2022, indicates a legislative trend toward requiring such labeling in the future.
MoCRA establishes federal standards for cosmetic business registration, product listing, recordkeeping, adverse event reporting, safety substantiation, GMP, recalls and more. It also requires the U.S. Food and Drug Administration (FDA) to formulate and publish regulations on fragrance allergen labeling. A list of fragrance allergens and specific labeling requirements will be published by the FDA in the future.
Canada
Currently, Health Canada (HC) does not require cosmetics to be labeled with fragrance allergens on the label. However, in February 2023, HC proposed amendments to require the labeling of 24 identified fragrance allergens in the ingredient list on the package of cosmetics when their concentration exceeds 0.001% in leave-on products or 0.01% in rinse-off products. The 24 allergens are consistent with those listed by the EU (refer to Table 4) and also subject to change by the EU.
This would make it easier for individuals with allergies to identify and avoid potential allergens in these products, which improves consumer safety. In addition, it would provide consistency in labeling requirements.
It is important to note that the proposed regulations are not yet finalized and may be subject to change based on feedback from stakeholders and the public. Nevertheless, if implemented, they would represent a significant step toward improving the safety of cosmetic and personal care products sold in Canada.
Other Countries
Finally, fragrance allergen labeling is not currently required in Hong Kong (SAR of China), China’s Taiwan, Japan or ASEAN.










