
Formulation work is exciting in that it seeks to develop new technologies that improve upon the performance of cosmetic products. Knowing and attending to safety, consumer preferences and conformance to global regulations is typically part of this process. For example, formulations may need minor alterations to conform to the requirements of specific countries or regions, although their framework generally remains constant.
Log in to view the full article
Formulation work is exciting in that it seeks to develop new technologies that improve upon the performance of cosmetic products. Knowing and attending to safety, consumer preferences and conformance to global regulations is typically part of this process. For example, formulations may need minor alterations to conform to the requirements of specific countries or regions, although their framework generally remains constant.
Reformulation to meet regulatory mandates is never as much fun as new product development. It not only requires the involvement of legal teams, but also can give rise to new technical issues. As such, chemists are sometimes challenged to maintain product performance while replacing ingredients, systems and/or procedures that have been routinely used in cosmetic products – and recent regulatory activity in the European Union (EU) has significantly impacted ingredients that are key components of established frameworks for color cosmetics: cyclic silicones and microplastics.
To understand these regulatory decisions, it is first helpful to consider a major difference between the U.S. and EU regulation of cosmetics, which is procedural. In the United States, cosmetics and other products are regulated by the U.S. Food and Drug Administration under the Food, Drug and Cosmetic Act, codified in the Code of Federal Regulations Title 21 (21CFR). They are exempt from U.S. Environmental Protection Agency (EPA) requirements under the Toxic Substances Control Act (TSCA) – the law regulating chemical substances.
In the EU, cosmetic ingredients are regulated by both the European Chemicals Agency (ECHA) under the chemical law EC No. 1907/2006, as amended, Registration, Evaluation, Authorization and Restriction of Chemicals (REACH); and the law specific to cosmetics, Regulation EC No. 1223/2009, as amended. The result in the EU is that both agencies have more routine impact on the regulation of the personal care industry. It is the former, REACH, that is driving activities around these two types of ingredients.
More specifically, D4, D5 and D6 cyclic silicones and microplastics – materials that have made significant contributions to the aesthetics and performance of color cosmetics – are facing bans under REACH. This article explains what these materials are, how they are used, why they were flagged and what banning them means for color cosmetic formulating.
Cyclic Silicones
The silicones affected by the bans are cyclic, meaning they have a ring structure, and they are volatile, easily evaporating at skin temperature. Silicone polymers are described by the number and type of silicon atoms units per molecule, x+y = 4, where R represents organic groups:
M unit = R3SiO
D unit = R2(SiO)2
T unit = R(SiO)3
Q unit = (SiO)4
Straight chain linear silicones, R = methyl (INCI: Dimethicone), are composed of D units ended by M units. Simple cyclics are composed entirely of D units arranged in a ring structure, named by the number of D units in the ring (i.e., tetra, penta, hexa, etc.). To avoid confusion, Table 1 gives the various names used to describe the cyclic compounds that are affected by the new EU regulations.
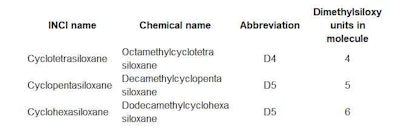 Table 1. Cyclic Silicones Affected by EU Regulations
Table 1. Cyclic Silicones Affected by EU Regulations
Cyclic dimethylsiloxanes have been used in cosmetics since the 1970s.1, 2 Their low odor, good chemical stability, minimal skin penetration – due to a bulky molecular size and shape – and low incidence of skin irritation favor their application in leave-on products. Originally, the INCI name Cyclomethicone referred to a mixture of cyclics. Later, they were differentiated into individual names for the four-, five- and six-member ring compounds; respectively: Cyclotetrasiloxane, Cyclopentasiloxane and Cyclohexasiloxane.
Decamethylcyclopentasiloxane has generally been the choice for a wide range of color cosmetic formulas for its moderate volatility, allowing adequate emolliency for spreading and blending, then evaporating to leave a long wearing film of color. For use in anhydrous lip and eye color, decamethylcyclopentasiloxane offers some compatibility with organic compounds. It functions as the vehicle for wax-containing anhydrous lip and eye formulas with only a small addition of non-volatile oil as the co-solvent for silicone film formers and elastomers that form films, contributing long-wear properties.
In addition, as a carrier, decamethylcyclopentasiloxane enables the incorporation of other useful ingredients into color cosmetics, including w/o emulsifiers, silicone resins, silicone elastomers and organomodified clay stabilizers. As a result, decamethylcyclopentasiloxane has often been the first or second ingredient by percentage in many of the w/o emulsions that have dominated the liquid/cream foundation market.
As an alternative, the lower volatility of dodecamethylcyclohexasiloxane has been preferred by some formulators. This property can reduce drying effects on the face and potentially lower health and environmental risk.
The cosmetic use of octamethylcyclotetrasiloxane has been avoided to a large extent in the United States following toxicological concerns from reported adverse effects following chronic inhalation studies in female rats.3, 4 Silicone suppliers do not promote octamethylcyclotetrasiloxane for cosmetic use; in fact, many cosmetic manufacturers require certification that raw materials containing other silicones are virtually free of the material.
Toxicological and Environmental Status
Toxicological and environmental behavior studies on the cyclic silicones are continually conducted and reviewed to supply the required data for registration under the chemical laws of many regions including Canada, the U.S. and the EU. Review of data by the regulators has been the basis for decisions regarding the status of all chemicals. Although cosmetic use is exempt from U.S. chemical law, the Toxic Substances Control Act, the cyclic silicones were reviewed by the U.S. Environmental Protection Agency for all other applications.
The EPA and Health Canada came to opposite conclusions from the EU regarding the environmental and toxicological properties of cyclic silicones. The EPA concluded that decamethylcyclopentasiloxane does not bioaccumulate due to its evaporation from the skin and exhalation,5 and it does not pose a risk to the environment.6 Health Canada noted that because cyclic silicones are volatile, they are excreted into the atmosphere, where degradation occurs – first to silanols, then to carbon dioxide, water and silicon dioxide (silica). Thus, these products do not present a danger to the environment.
Furthermore, the amount that volatilizes and is degraded in the atmosphere is equal to new emissions, evidenced by the measured constant amount in the environment, so per Health Canada, the material is persistent but does not accumulate. The material has therefore been deemed not harmful to human health or the environment.7
In contrast, in the EU, under Annex XVII to (EC) No.1907/2006 (REACH), octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane were both deemed as very persistent and very bioaccumulative.8 ECHA issued the Annex XV Restriction Report proposal to ban the presence of octamethylcyclotetrasiloxane, decamethylcyclosiloxane and dodecylcyclohexasiloxane in cosmetic products at ≥ 0.1%, effective Jan. 31, 2022, according to Commission Regulation (EU) 2018/35, amending Annex XVII of Regulation (EC) No.1907/2006.9
On June 23, 2023, the EC European Commission notified the World Trade Organization of the intent to amend Annex XVII of Regulation EC1907/2006, to enact a similar percentage restriction to octamethylcyclotetrasiloxane, decamethylcyclopentasiloxane and even dodecamethylcyclohexasiloxane, “as used in various consumer and professional products, including cosmetics.”10, 11
This move was based on the European Chemical Agency’s (ECHA’s) conclusion that all cyclics were considered substances of very high concern (SVHC), with persistent, bioaccumulative and toxic properties under REACH. For leave-on cosmetics, the ECHA proposed the ban would go into effect for leave-on cosmetics three years after posting, “in view of the difficulty in reformulating.” Although it was stated in the notification to be anticipated by the end of 2023, as of this writing, the restriction (virtual ban) in leave-on cosmetics has not been finalized.
Alternatives to Cyclic Silicones
In terms of reformulating, there are no 1:1 replacements for cyclic silicones. And because they are not just a single ingredient in a formula, but also the solvent or carrier for many of the other ingredients, their upcoming ban is affecting many ingredient choices. Not only must the formulator who is using cyclics choose a replacement solvent as a carrier in the formulation, so must the suppliers of the many emulsifiers, film formers, texturizers and thickeners sold as dispersions or solutions in cyclic silicones find suitable alternatives.
When attempting to choose alternates, some of which are described below, careful evaluations of performance, pharmacological/toxicological effects, solvency, interactions with packaging components and physical stability in use must be performed with the full range of finished goods involved.
Low molecular weight, branched chain esters: These materials can duplicate the dry skin feel of cyclic silicones but not their volatility, and the evaporation of a portion of the fluid vehicle is necessary to leave a long wearing, rub-proof color film on the skin.
Linear low molecular weight silicones: 1 cSt, 1.5 cSt and 2 cSt, silicone blends can duplicate some properties of the cyclics but multiple trials are usually required to duplicate existing formulations. In addition, some formulators are bypassing linear siloxanes as cyclic replacements because there is concern that the ECHA could propose extending regulatory restrictions to the straight chain compounds.
Volatile hydrocarbons: These materials have a long history of use in personal care, equal to that of the cyclic silicones, but volatile hydrocarbons can be expected to pose a higher potential for skin irritation due to their greater solubility in epidermal protective lipids. Examples of synthetic hydrocarbons include isododecane, C11-13 hydrocarbons and tridecane; naturally derived options include coco alkanes.
Additional considerations when using volatile hydrocarbons include:
- Differing interactions with other formula ingredients. Silicones and hydrocarbons are both non-polar but hydrocarbons are better solvents for other compounds based on organic (carbon) chemistry. They offer improved solubility will lower solidification points and soften or lower the viscosity of wax-containing products.
- Reformulation, rather than simply replacing an ingredient, may be necessary. In fact, a completely different formulation approach may be required – particularly for emulsion formulations due to altered interactions with emulsifiers, gellants, waxes and film formers.
- Primary emulsifiers for w/o formulations are often specific to the type of oil(s) in the external oil phase. A PEG-x/PPG-y dimethicone emulsifier that forms stable water in silicone emulsions in cyclopentasiloxane may have insufficient compatibility with a volatile hydrocarbon to produce a stable emulsion. An alkyl (e.g., cetyl) PEG-x/PPG-y dimethicone or a polyglyceryl-X polyisostearate or ricinoleate type emulsifier may be more suitable for water in hydrocarbon emulsions.
- Time-consuming stability, pharm-tox and performance (i.e., appearance and wear) tests are required at each stage of product development.
- Volatile hydrocarbons have poor compatibility with some common packaging materials, particularly the hydrocarbons, polyethylene and polypropylene. High weight loss, deformation or stress cracking are evidence that alternate materials are needed for at least some components of a package.
If naturally derived alternatives are desired, those chosen must be as volatile as the synthetic cyclic siloxanes to achieve similar effects. Since the chemical properties of synthetic and naturally derived volatile hydrocarbons are the same, they will have the same advantages and disadvantages. And while there are no natural volatile hydrocarbons, there are several that fit the description of naturally derived if the starting chemical source is other than petroleum.
Microplastics
The contamination of oceans, lakes and streams with large amounts of fine plastic particles is a worldwide concern. This has brought about efforts to control emissions from many product types including cosmetics; especially those that are rinse-off cleansing products that contain insoluble scrubbing beads – previously referred to as microbeads but more recently, microplastics. Over the last ten years, industry and regulators have responded to reduce the contribution of personal care products to plastic pollution.
2014: In 2014, the U.S. cosmetics industry voluntarily announced a phase out of microbeads used as exfoliants in rinse-off cleansers to reduce the pollution from waste-water effluent.12 European companies did similarly.13 Spherical polyethylene beads used for this purpose were the primary materials affected.
2015: In addition, the U.S. Congress passed the Microbead Free Water Act,14 effective on July 1, 2017, prohibiting the manufacture of rinse-off products containing microbeads.
2017: In 2017, the EU Parliament tasked the ECHA with preparing a dossier assessing the potential need for restricting the use of all “microplastic” materials.15 In this request the EU legislators expanded the scope of materials of concern from microbeads to all microplastics, defined as “synthetic, water insoluble polymers of 5 millimeters (mm) or less (‘synthetic polymer microparticles’) of any dimension that are present in products to confer a sought-after characteristic (‘intentionally present’).”
The goal was to address any risk that microparticles may pose to the aquatic environment (“the Annex XV dossier.”) The revised scope of the concern from microbeads used as exfoliants to polymeric microparticles having the given defined characteristic greatly extends the type of raw materials to include many.
2018: One year later, the EU Parliament requested a ban on microbeads from the European Commission.16 From March to May 2018, stakeholder input was accepted by ECHA.
2019: On Jan. 29, 2019, ECHA published the Annex XV Restriction dossier17 to address the estimated 42,000 tonnes of microbeads eventually released to the aquatic environment per year. Microbeads as abrasives were expected to be voluntarily phased out by 2020 but the scope of the proposed “restriction” – effectively, a ban – was expanded to include both rinse-off and leave-on products and not limited to those used as exfoliants.
If proper disposal could be documented, ingredient restrictions could be derogated (waived), so long as those disposal instructions were provided. The many spherical polymers used for texture and appearance modification in leave-on skin care and color cosmetics were included in the proposed ban on microplastics.
Note that microbeads referred to scrubbing beads in wash-off products, whereas microplastics became the new definition in the 2019 proposal banning all plastic particles in cosmetics. A four- or six-year transition period was proposed for cosmetics, and a five- or eight-year transition period for encapsulated fragrances; stakeholder input was accepted by ECHA from May to September 2019.12
The final EU definition of microparticles is synthetic polymer particles or polymer-coated particles below 5 mm in all dimensions that are organic, that are solid, porous or hollow – widely used as fillers, texture modifiers and light diffusers in color cosmetics. This definition includes fiber-like particles 0.3 μm-15 mm in length/length, to a width ratio > 3.19
Applications of such materials are far wider than cleansing products and cover the range of color cosmetics: lip color, pressed and loose powder, foundation and blush, eye color, emulsion foundations, anhydrous foundations, mascara and nail lacquer, among others. The particles absorb oil and provide slip and a dry, non-greasy skin feel.
In pressed powders, some act as press aids and are used to increase pick-up and slip, and to soften skin feel during application. Some also diffuse light, which is particularly useful in facial products, providing a soft-focus effect to obscure skin imperfections.
It is clear that to comply with the ban, an enormous number of products will require reformulation to duplicate skin feel and performance.
2020: On June 23, 2020, the ECHA Committee on Risk Assessment (RAC) agreed that action was necessary, that no lower percentage limit should be set, and that testing should be required to prove claims of degradability.18 On Dec. 10, 2020, the ECHA Committee for Socio-economic Affairs (SEAC) submitted opinions of the economic impact of the proposed restrictions.18
2023: On April 20, 2023, EU member states voted in favor of the restrictions on microplastics, then on Sept. 25, the Commission issued Regulation (EU) C(2023)6419 amending REACH Annex XVII to (EC)No. 1907/2006, regarding intentionally added synthetic polymers (microplastics), was adopted. The applicable effective date was Oct. 17, 2023.19
Rinse-off exfoliants and glitter were banned immediately. The recall of products already on the market was not required. The effective date for leave-on products and encapsulated fragrances is Oct. 17, 2029, while the effective date for lip, nail and makeup products is Oct. 17, 2035, in recognition of the extent and difficulties involved with the replacement of microplastics. After Oct. 17, 2031, the packaging of any microplastic products remaining must include the statement “contains microplastics.19
Replacing Microplastics
Replacing microplastics in formulations means using natural or non-organic (non-carbon-containing) alternatives. Silica spheres are the most obvious choice, as they are inorganic; and non-organic polymer spheres are permitted. Solid, porous and hollow silica spheres are already formulated into color cosmetics – but they do demonstrate many properties that differ from polymeric spheres in terms of oil absorption, density, bulk density, opacity, slip, crush, friability and skin feel: soft vs hard.
Other natural or naturally derived materials available in spherical form include cellulose, polylactic acid and Copernicia cerifera (carnauba) wax spheres. A variety of starches, although not spherical, also can provide useful slip and visual effects in pressed powders.
When replacing known filler materials, the first step is to compare data specifications of the original material with the alternative. Obvious differences such as oil absorption or loose bulk density could be the basis for starting with a modification to the standard formula.
Prepare a batch of the existing formula at the same time as – and with the same lab equipment as – the experimental batch. Evaluate as usual, then determine if formula adjustments can be made to more closely duplicate the original.
Once an alternative formulation is chosen, it will be important to carefully evaluate the short- and long-term stability of formulas under a variety of conditions. If natural products are chosen, remember that they tend to be less resistant to microbial contamination than their synthetic counterparts. The replacement of polymeric microspheres will be an ongoing effort and the scope of the ban is still being assessed by the personal care industry.
Conclusions
The described regulatory bans will seriously affect color cosmetic formulation. As such, replacing cyclic silicones and plastic microplastics have been the focus of much formulation and many related cosmetics R&D activities, both in the short and long term. Formulation chemists are hoping for some relief as new chemistries and processes are developed – with the requirements for long term human and environmental safety being more clearly codified in the EU.
References
1. Goldner, T., Fotiu, E. and Tietjen, M. (1984, Feb 14). Cosmetic compositions. U.S. Patent 4,431,673. Assigned to Revlon, Inc. Available at https://patents.google.com/patent/US4431673A/en
2. Davy, P.F. and Drolet, M.L. (1978, Nov 21). Cosmetic stick. U.S. Patent 4,126,679. Assigned to Armour-Dial, Inc. Google Books. Available at https://www.google.com/books/edition/Index_of_Patents_Issued_from_the_United/Su7ty2FNwmUC?hl=en&gbpv=1&bsq=4126679
3. Burns-Naas, L.A., Meeks, R.G., … Thevenaz, P., et al. (2002). Inhalation toxicology of octamethylcyclotetrasiloxane (D4) following a 3-month nose-only exposure in Fischer 344 rats. Intl J Toxicol, 21(1), pp 39-5.
4. Siddiqui, W.H., Stump, D.J., Plotzke, K.P., Holson, J.F. and Meels, R.G. (2006). A two-generation reproductive toxicity study of octamethylcyclotetrasiloxane (D4) in rats exposed to whole body vapor inhalation. Reprod Toxicol, 23(2) pp. 192-201.
5. De Kant, W. and Klaunig, J.E., eds. (2016, Feb). Toxicology of decamethylcyclopentasiloxane. Regulatory Toxicology and Pharmacology, vol 74, suppl, pp. 67-76, available at https://pubmed.ncbi.nlm.nih.gov/26111607/
6. Mackay, D., Cowan-Ellsberry, C.E., ... Kim, J., et al. (2015, 24 Jul). Decamethylcyclopentasiloxane (D5) environmental sources, fate, transport and routes of exposure. Envt Toxicol and Chem. Available at https://setac.onlinelibrary.wiley.com/doi/full/10.1002/etc.2941
7. Government of Canada. (2012, Feb 25). Canada Gazette, Part 1, Vol. 146, Number 8: Government Notices. Available at https://gazette.gc.ca/rp-pr/p1/2012/2012-02-25/html/notice-avis-eng.html
8. SGS SA. (2019, Mar 7). New restriction proposals. Available at https://www.sgs.co.uk/-/media/local/uk/documents/brochures/sgs-crs-reach-newsletter-issue-34-a4-en-19-04.pdf
9. Ibid
10. REACH24H. (2023, Jun 29). EU proposes stricter restrictions on D4, D5 and D6 in cosmetics. Available at https://www.reach24h.com/en/news/industry-news/cosmetic/eu-proposes-stricter-restrictions-on-d4-d5-and-d6-in-cosmetics.html.
11. ToxPartner. (Accessed 2023, Mar 4). Ban on certain CMR substances in consumer products from 1 December, 2023. Available at https://www.toxpartner.com/articles/ban-on-certain-cmr-substances-in-consumer-products-from-1-december-2023
12. PCPC. (Accessed 2024, Mar 9). Microbeads. Available at https://www.personalcarecouncil.org/public-policy/microbeads/
13. L'Oreal. (Accessed 2024, Mar 9). Microplastics in cosmetic products - let's mythbust. Available at https://inside-our-products.loreal.com/our-approach/microplastics-cosmetic-products-lets-mythbust
14. Pallone, F., Jr. (2015, Mar 4). H.R. 1321 - Microbead-free Waters Act of 2015. Available at https://www.congress.gov/bill/114th-congress/house-bill/1321
15. European Commission. (2017, Sept 11). Note for the Attention of Mr. G. Dancet, Executive Director, ECHA. Available at https://echa.europa.eu/documents/10162/17233/microplastics_cion_reqst_axvdossier_en.pdf
16. European Parliament. (2018, Sep 13). European Parliament resolution of 13 September 2018 on a European strategy for plastics in a circular economy (2018/2035(INI). Available at https://www.europarl.europa.eu/doceo/document/TA-8-2018-0352_EN.html
17. ECHA. (2019, Aug 22). Annex XV restriction report. Proposal for a restriction – Intentionally added microplastics. Available at https://echa.europa.eu/documents/10162/05bd96e3-b969-0a7c-c6d0-441182893720
18. ECHA (2020, Dec 10). Committee for risk assessment (RAC) and committee for socio-economic analysis (SEAC): Opinion on an annex XV dossier proposing restrictions on intentionally-added microplastics. Available at: https://echa.europa.eu/documents/10162/a513b793-dd84-d83a-9c06-e7a11580f366
19. European Commission. (Accessed 2024, Mar 9). Commission Regulation (EU) 2023/2055 - Regulation of microplastics intentionally added to products. Available at https://single-market-economy.ec.europa.eu/commission-regulation-eu-20232055-restriction-microplastics-intentionally-added-products_en










