
Read parts I and II of this article, adapted into one, in the September 2023 digital magazine.
Part I in this series on preservative efficacy testing for w/o cosmetic formulations explained the relevant formulation conditions that determine a potential microbial contamination. It also considered method modifications that should be considered when carrying out microbial tests in products that contain water in their internal phase. These particular product characteristics limit the explanatory power of preservative efficacy test (PET) results, influencing the microbiological safety assessment.
The PET, in principle, is an essential criterion for microbiological product safety assessment (see European Cosmetics Regulation1) but it cannot and should not be understood as the only evaluation criterion. On the contrary, safeguarding a product's safety must be assured by applying various measures that are to be verified in the course of the safety assessment. This requirement is increasingly important in the case of w/o products due to limited conclusiveness of PET results due to the known method limitations.
A reliable safety assessment is only possible with a systematic, holistic approach.2, 3 The microbiology specialist must draw conclusions from the test results, relevant additional findings and influencing factors in order to make a product-specific assessment. This article summarizes the relevant aspects in the case of w/o products. Here, reference is made to explanations in Part I without repeating the details or literature references.
Practical Experience with Microbiological Stability of W/O Emulsions
An assessment of the Safety Gate (formerly RAPEX) data4 for cosmetic product recalls associated with microbiological issues in the last few years (2011-2020) revealed that of some 108 recalled cosmetic products, at most, nine cases (8%) may have been related to w/o type cosmetics. Notably, the assignment of product type was based only on the type of emulsifier or application, since data reported generally do not give exact information on the product type. In any case, this small quantity only serves as an indicator for trends because a statistical reference value for the percentage of w/o formulations in the market is lacking. Nevertheless, w/o-based products have a long history of use in the cosmetic industry and are known to have a marginal microbiological risk.
A compilation of microbiological test results from a contract laboratory between 2018 and 2020 (data kindly provided by BAV Institute, Offenburg, Germany) shows that 17% of all w/o type products tested failed the acceptance criteria according to DIN EN ISO 11930;5 for non-w/o products, this is the case for only 5% (see Figure 1a). Given the methodological difficulties and fluctuations in PET results with w/o emulsions, it is not surprising to see this increased failure frequency in PET results.
Furthermore, w/o samples did not exceed microbial limits more frequently, per tests according to DIN EN ISO 17516,6 than non-w/o samples (see Figure 1b). This data indicates that w/o products do not necessarily exhibit increased microbiological safety problems, although they fail the PET more frequently than other product types. The lack of a suitable test, as described in Part I, may explain these results.
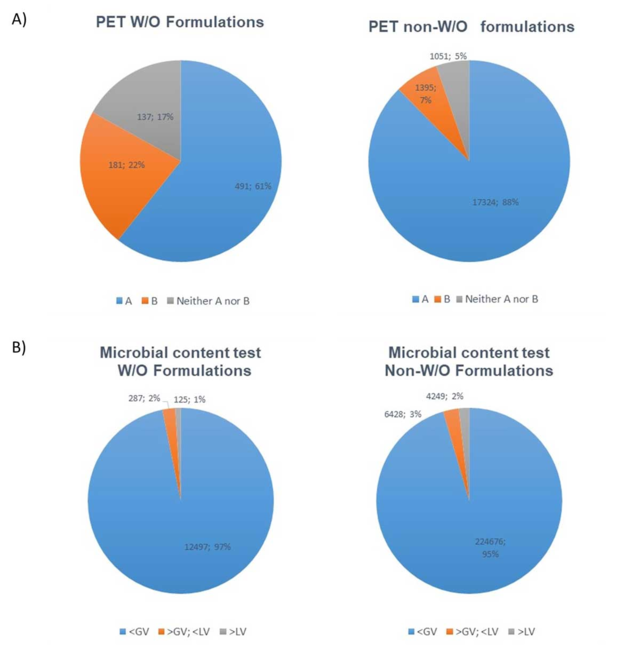 Figure 1. A comparison of microbiological test results for w/o formulations and non-w/o formulations (BAV Institute years 2018/2019/2020) for:
A) Logarithmic reduction criteria for PET (according to DIN EN ISO 11930): criterion A/criterion B or failure to meet neither criteria A or B
B) Microbial content tests (according to DIN EN ISO 17516,6 where the microbial content: complies with guide value (< GV); does not comply with guide value (> GV; < LV); or does not comply with limit value (> LV).
Limit value (LV) = specifications of the Norm; guide value (GV) is 10% of the LV
Figure 1. A comparison of microbiological test results for w/o formulations and non-w/o formulations (BAV Institute years 2018/2019/2020) for:
A) Logarithmic reduction criteria for PET (according to DIN EN ISO 11930): criterion A/criterion B or failure to meet neither criteria A or B
B) Microbial content tests (according to DIN EN ISO 17516,6 where the microbial content: complies with guide value (< GV); does not comply with guide value (> GV; < LV); or does not comply with limit value (> LV).
Limit value (LV) = specifications of the Norm; guide value (GV) is 10% of the LV
Proper Testing Procedure
The recommended method for testing water-miscible cosmetic formulations according to DIN EN ISO 11930 incurs several problems when applied to non-water-miscible formulations such as w/o emulsions. Empirical evidence shows low reproducibility or fluctuations in the results. Method modifications for this type of product could, at least to certain extent, reduce such fluctuations (see Part I).
In the case of fluctuating PET results for w/o products, it can be useful to increase the number of samples to verify the results. Several tests should be conducted during the development process using the same defined method.
Improvements also can made in the accuracy of microbial counts by not only appropriate mixing or further method modifications, but also by increasing the number of samples taken at each time point. An averaged result can additionally contribute to controlling fluctuations. In any case, most importantly, trends for microorganism proliferation must not be present, which may be a complicated task with fluctuating PET results.
If fluctuations in PET results are already present during early development stages of a w/o formulation, one might also consider reviewing the PET method being used. In terms of testing the final product control for approval, an increased number of samples distributed over the batch should be employed for the most reliable results.
Preservative Effect in W/O Emulsions
The preservation system of w/o-based cosmetic products generally contains two different hurdles that can effectively interact: 1) limited water availability in the emulsion and 2) the use of substances with antimicrobial effects; i.e., allowed preservatives according to Annex V of the Cosmetics Regulation.1
It is paradoxical that the evaluation of an antimicrobial effect due to limited water availability requires a test procedure that adds microorganisms to water in a microbial suspension. For testing, these introduced microorganisms are present as extrinsic contamination in non-dispersed water droplets.
Conversely, the option of using an oil-soluble carrier system, e.g., mineral oil, to limit "added" added water in the test has additional drawbacks. Hence, depending on the microorganism inoculated, the carrier itself may have an inhibitory effect,7, 8 or it may have an impact on the emulsion system. A comparable situation where a PET is not applicable includes other water-free formulations or formulations with low water activity (see "low risk products" according to DIN EN ISO 29621).9
Since microbial control by means of limited water availability depends on the very specific chemical-physical conditions and stability of every formulation (see Part I), adding antimicrobial substances or other ingredients with antimicrobial effects in w/o formulations is a common approach to safeguard preservative effects. In many cases, a combination of various ingredients can boost the microbial inhibitory effect.
The antimicrobial activity of preservative substances in formulations with limited water availability depends largely on the polarity of the substance and their location within the emulsion. In order for antimicrobial substances to interact with microbial cells, it is necessary for a sufficient concentration of the preservative (or supporting ingredients, if necessary) to be available in the aqueous parts of the emulsion.10
As many preservatives are partially or completely lipophilic, higher concentrations accumulate in the continuous phase of an emulsion and are only sparingly soluble in the water phase (partitioning effect, bioavailability). This exposes a challenge for formulators, as the emulsion must be designed so that the antimicrobial additives do not migrate to any other phase that is thermodynamically more favorable than the aqueous phase.
Therefore, for testing the preservative effectiveness of antimicrobial substances in w/o formulations, the PET method is limited due to test microorganisms sitting as extrinsic contamination on the lipophilic phase, isolated from the aqueous phase of the emulsion where the antimicrobials could work. Additionally, the coalescence of water drops and random distribution of active substances result in an uneven distribution of antimicrobial concentrations throughout the product.
If preservative substances are added to w/o formulations to reduce potential microbial risks during manufacturing, e.g., intrinsic contaminants in the dispersed water phase, the question arises of whether the microbial reduction requirements according to DIN EN ISO 11930 (PET) are justified in all cases. Particularly for stable emulsions, with smaller-diameter water droplets, a low tendency for flocculation and/or coalescence, and high viscosity, it is reasonable to consider lenient PET acceptance criteria, as the risk for microbial proliferation is considerably reduced by the smaller water droplets.11 The antimicrobial effect of the emulsion microstructure, in this case, contributes to the microbial stability of the product.
Conversely, emulsion instabilities increase the importance of preservative additives for microbial control and more rigorous PET acceptance criteria should be set. The confirmation of PET results is a basic requirement, particularly when the defined methods and acceptance criteria are set for specific products and deviate from the guidelines. Accordingly, evaluations should be made through the surveillance of practical conditions; e.g., market monitoring, microbial content of complaint products, internal controls, etc.3 Additionally, in-use studies can contribute largely to the microbial risk assessment of the product by giving information about the suitability of the package and the mode of application.12
Securing Specific Product Properties
As stated, the characteristics of w/o formulations have a significant influence on microbial test results, on PET performance/reproducibility, and on microbial content tests—and consequently, on a reliable microbial risk assessment. Every w/o-type product has a different chemical-physical emulsion parameters, e.g., phase volume distribution, droplet size, rheology, etc., which are given by the formulation and manufacturing process.
These aspects are important to consider for the evaluation of microbial contamination risks, extrinsic as well as intrinsic contamination, and setting testing and evaluation criteria. Furthermore, compliance with cosmetic GMP (DIN EN ISO 22716) ensures a standard of product characteristics and additional quality controls.13
Finally, in addition to PET results, a reliable product-specific safety assessment should consider the following aspects:
- Establish appropriate microbiological purity requirements for production and release purposes (aerobic plate counts and exclusion of specific microorganisms).
- Ensure continuously stable w/o emulsions via suitable formulation of chemical-physical parameters, production and quality control test parameters. Again, microbiological safety depends on product parameters.
- Ensure sufficient hygiene measures in the manufacturing processes,14 with the aim of avoiding intrinsic contamination.
- Make and collect product usage and application notes, if applicable.
References
1. European Commission (2009). Regulation of the European Parliament and of the Council on Cosmetic Products. Available at https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32009R1223&from=EN
2. Eigener, U. (2020). Safeguarding microbiological quality and safety of cosmetic products through a system approach. SOFW-J 146 (1+2), 42-46.
3. Eigener, U. (2021). VIII.1 Mikrobiologische qualität und produktsicherheit kosmetischer mittel. In: Baumgart, J., Becker, B. and Stephan, R., eds., Mikrobiologische Untersuchung von Lebenmitteln. Hamburg, Germany. Behr’s Verlag.
4. European Commission (accessed 2020, Apr 29). Safety Gate search alert, European Union, 2005–2021. Available at https://ec.europa.eu/safety-gate-alerts
5. International Organization for Standardization (2019). Cosmetics—Microbiology—Evaluation of the antimicrobial protection of a cosmetic product (ISO 11930). Available at https://www.iso.org/standard/75058.html
6. International Organization for Standardization. (2014). Cosmetics—Microbiology—Microbial limits (ISO 17516). Available at https://www.iso.org/standard/59938.html
7. Personal Care Product Council. (2016). Microbiology guidelines. Section 20: M-6 Method for preservation testing of atypical cosmetic products. Available (for purchase) at https://webstore.ansi.org/Standards/PCPC/CTFAMicroGuidelines?source=preview
8. Yablonski, J.I. (2006). Risk factor assessment of anhydrous atypical cosmetic products. In: Orth, D.S., Kabara, J.J. and Denyer, S.P., eds, Cosmetic and Drug Microbiology. Boca Raton, FL USA. CRC Press pp 133-144.
9. International Organization for Standardization. (2017). Cosmetics—Microbiology—Guidelines for the risk assessment and identification of microbiologically low-risk products (ISO 29621). Available at https://www.iso.org/standard/68310.html
10. Ibid Ref 8, pp 329-331.
11. Brocklehurst T.F., Parker M.L., Gunning P.A., Coleman H.P. and Robins, M.M. (1995). Growth of food-borne pathogenic bacteria in oil-in-water emulsions: II— Effect of emulsion structure on growth parameters and form of growth. J Appl Bacteriol 78(6) 609-15.
12. Eigener, U. (1999). Produktstabilität auf dem Prüfstand: Konservierungsbelastungstests für kosmetische Mittel. Parf Kosm 80 36-4.
13. German Cosmetic, Toiletry, Perfumery and Detergent Association (2016). IKW: I.9.1 Kosmetik- GMP–gute Herstellungspraxis–Die Norm DIN ISO 22716. In: Reinhart, A., eds, Praxishandbuch kosmetische Mittel. Hamburg, Germany. Behr’s Verlag.
14. German Society for Scientific and Applied Cosmetics. (2010). DGK. Industrial Hygiene in the Cosmetics Sector. Augsburg, Germany. Verlag für chemische Industrie, H. Ziolkowsk. Available (for purchase) at https://www.sofw.com/de/shop/buecher/product/327-dgk-industrial-hygiene-in-the-cosmetics-sector










