Sulfates and sulfonates are both classes of anionic surfactants. Both classes commonly are used in personal care applications. However, there are many salient differences including their structure, function and the raw materials used to make them.
Sulfates
Sulfates are compounds that possess a carbon, oxygen sulfur bond. They include things like sodium lauryl sulfate and sodium laureth sulfate.
The presence of the C-O-S bond makes these materials hydrolyze at low pH values, producing alcohol and inorganic sulfate. Sulfates are stable in base at high pH values and, if ethoxylated to a relatively high level, produce low foaming alkali soluble surfactants. Sulfates are made by the reaction of fatty alcohol or fatty alcohol ethoxylates with reagents like SO3 or chlorosulfonic acid (CSA).
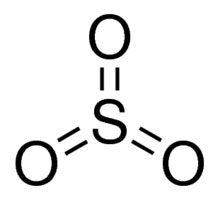
SO3
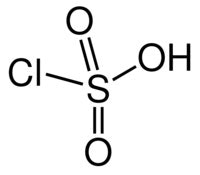
CSA
Sulfonates
Sulfonates are compounds that possess a carbon sulfur bond. They include things like alpha olefin sulfonate (AOS).
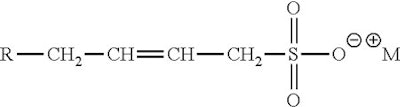
AOS
This bond is stable at a much wider range of pH values and can therefore be used in acidic environments. Alpha olefin sulfonates are made by reaction of alpha olefin and SO3.










!['Green is good but maybe not good enough. Future aspects will focus on more and additional aspects ... taking a look at the entire product lifecycle [and] looking at the entire value chain ...'](https://img.cosmeticsandtoiletries.com/files/base/allured/all/image/2023/08/Evonik_Value_Chain_Video_Interview_Sept_2023.64ecb11af2ee6.png?auto=format%2Ccompress&fit=crop&h=191&q=70&rect=264%2C115%2C1130%2C634&w=340)