The attitudes and perception of cosmetic preservatives have undergone significant changes in recent years. Traditional preservatives such as parabens, thiazolinones, formaldehyde-releasers and organic halogens have come under scrutiny, and, consequently, there is growing interest in alternative means of preservation with other antimicrobial choices; for example, organic acids and aromatic alcohols. This also follows a demand for milder and more natural ingredients. The use of alternative antimicrobials and the claim preservative-free have become popular in today’s cosmetics market as well, the latter thought to be due in part to current consumer beliefs that a product containing preservatives may pose a higher risk than “unpreserved” or “self-preserved” options.
In many formulations, “preservatives” have been replaced by cosmetic ingredients with one or more specified purposes that have the added benefit of preserving the formulation. To some, this raises controversy, as these are not officially classified as preservatives, and do not require labelling as such. The antimicrobial activity of plant oils and extracts, for example, has been recognized for years, together with other functional activities, i.e., lenitive, antioxidant, etc.1 Regardless of an ingredient’s classification, cosmetics on the market must be self-preserving or well-preserved.
Various aldehydes and alcohols, aromatic and aliphatic compounds, or terpenes and organic acids are among the most active molecules that can be used to reduce the levels of traditional preservative needed—or, in combination with other substances, replace them altogether.2 Regardless of an ingredient’s classification, microbial contamination leads to product deterioration, and can result in serious risks for consumer health; thus, cosmetics placed on the market must be either self-preserving or well-preserved.
The presented study investigates the antibacterial and antifungal activities of a blend of caprylyl glycol and phenethyl alcohol, referred to here as blend 1, in comparison with another composed of caprylyl glycol, glyceryl caprylate, glycerin and phenylpropanol, referred to here as blend 2. This blend was chosen for comparison for its preservation properties, and because it is based on a similar combination of caprylyl glycol with an aromatic alcohol; it also has the same suggested use range of 1.0–1.5%.
Phenethyl Alcohol and Caprylyl Glycol
Phenethyl alcohol is a nature-identical fragrance ingredient with a mild, flowery, rose-like scent that can mask the scent of other ingredients. It is used in cosmetics as a fragrance component, as well as in deodorants, food and flavoring agents, pharmaceuticals and ophthalmic solutions. In addition to its aroma, it has antimicrobial activity against bacteria, especially Gram-negative, and fungi.3 Phenethyl alcohol causes a rapid and reversible breakdown in the permeability barriers of bacterial cells. This alteration of membranes leads to the disruption of many intracellular functions and the inhibition of DNA synthesis.4
Caprylyl glycol is a C-8 aliphatic 1,2 diol with the suitable chain length to also destabilize and disrupt the microbial cell membrane.5, 6 Its antimicrobial properties have made it one of the most popular multifunctional ingredients for the replacement of traditional preservatives. It is also used as a skin conditioner, emollient and humectant agent in cosmetics.7
The combination of phenethyl alcohol with caprylyl glycol provides a synergistic antimicrobial effect, as will be shown, due to the wetting ability of caprylyl glycol, which may enhance the intracellular penetration of phenethyl alcohol. Both ingredients are globally approved for cosmetics without restrictions, and the mixture of caprylyl glycol and phenethyl alcohol has a favorable profile in terms of potential skin intolerances and adverse reactions.8, 9 These ingredients were chosen for their good antimicrobial action, as well as other favorable cosmetic functions. They are multifunctional ingredients, in line with the principles of self-preservation and “hurdle technology” of cosmetic products, as described extensively by Orth and Kabara.7 The wetting ability of caprylyl glycol may enhance the intracellular penetration of phenethyl alcohol.
The antimicrobial activity of phenethyl alcohol with caprylyl glycol also was studied in combination with chelating agents, to evaluate the potential effects on blends 1 and 2. The synergistic effect of EDTA with preservatives is well-known. Chelating agents, EDTA, lactic acid, citric acid and phytic acid increase the permeability of cell membranes of bacteria, thus making them more sensitive to antimicrobial agents. When used as part of a preservative strategy, chelating agents work synergistically with antimicrobial additives by controlling the metal ions that contribute to microbial cell growth and viability. A chelating agent rate of 0.1–0.3% can significantly reduce microbial contamination, resulting in increased product shelf life.10
Chelating agents also are known to enhance the preservative action of phenethyl alcohol and medium-chain C8-C12 compounds.11-13 However, environmental concerns have been raised14 in regards to complexing agents such as EDTA. Indeed, the environmental acceptability of EDTA and its salts are currently under debate as their biodegradability is poor. Therefore, tetrasodium glutamate diacetate and sodium phytate complexing agents were investigated, as they are obtained from natural sources and are readily biodegradable and, therefore, more ecological and sustainable solutions.
Materials and Methods
Ingredients: As noted, blend 1 was tested for its preservative activity in a variety of aqueous systems and emulsions, and blend 2 was used for comparison.
Antimicrobial activity: The antimicrobial activity of the blends against five common cosmetic contaminants was determined visually following the Minimum Inhibitory Concentration (MIC) and Minimum Biocidal Concentration (MBC) methods. These tests enable full determination of bacteriostatic/fungistatic and bactericidal/fungicidal activity, respectively. The MIC value is the lowest concentration of preservative that inhibits the visible growth of test organisms after 48 hr for bacteria, 72 hr for yeasts, and 5 days for molds. The MBC value is the lowest concentration of preservative that kills more than 99.9% of the initial concentration of microorganisms.
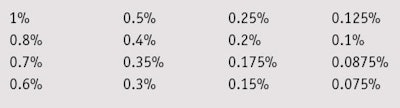
Microbial suspensions of single microorganisms, i.e., bacteria and fungi, from ATCC culture collection were prepared in suitable nutrient broths, and serial dilutions of the test samples were made in 96-well sterile micro-titer plates (see Table 1). The wells were inoculated with a single culture and microbial suspension to reach a concentration of 106 cells/mL, then plates were incubated at 35°C for 48 hr for bacteria and at 25°C for 5 days for fungi. The MIC and MBC of all mixtures were determined with and without chelating agents as preservative boosters. The chelating agents were added at various concentrations from 0.0125% to 0.2%, calculated as active matter. Due to the alkalinity and acidity of the complexing agents used, the final solutions of the mixtures were adjusted to pH 6.
Organisms: The following organisms were used for the tests described: Staphylococcus aureus ATCC 6538 (S. aureus), Pseudomonas aeruginosa ATCC 9027 (P. aeuriginosa), Escherichia coli ATCC 8739 (E. coli), Candida albicans ATCC 10231 (C. albicans) and Aspergillus brasiliensis ATCC 16404 (A. brasiliensis).
Inocula preparation: Bacteria were cultured on Tryptone Soya Agar (TSA) for 24 hr at 37°C. C. albicans and A. brasiliensis were grown on Sabouraud dextrose agar (SDA) at 25°C for 48 hr and 5 days, respectively. The microbial concentration of inoculum was 106/mL—opacity comparable to the 0.5% McFarland standard.
Challenge testing: Challenge tests were performed according to the Personal Care Products Council (PCPC) and European Pharmacopoeia methods. These are 28-day tests used to verify the effectiveness of a preservative system in finished personal care products. The test formulations are placed in sterile containers and inoculated with the test bacteria (i.e., S. aureus, E. coli and P. aeruginosa) and fungi (i.e., C. albicans and A. brasiliensis) as pure cultures at the onset of testing (0 hr). Then they were sampled at 48-hr and 7-day intervals for 28 days. A neutralizer was incorporated in the plated agar when recovering bacteria by streaking plates. The number of viable microorganisms per gram of product was determined by counting the colonies on the plates. A negative result, i.e., the absence of colonies, was reported at < 10 colony forming units (CFU)/g.
The challenge tests are considered to pass the PCPC and European Pharmacopoeia methods if the following criteria are fulfilled: regular and progressive reduction of the microbial load; and preservative effectiveness: bacterial reduction = 99% (2 Log) reduction 2 days after inoculation and 99.9% (3 Log) reduction 7 days after inoculation; and fungal reduction = 90.0% (1 Log) reduction 7 days after inoculation and 99% (2 Log) reduction 14 days after inoculation. Despite these evaluation criteria, it is generally agreed that inoculum should be significantly reduced by 48 hr, with following significant reductions after 7 days and no increase after 28 days.
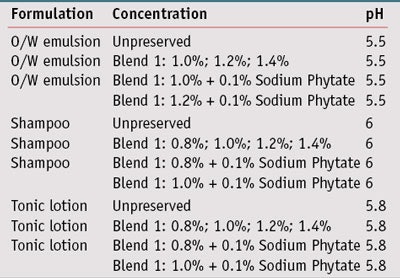
Cosmetic formulations: Results from the challenge testing led to the development of test formulations using blend 1, as it showed the best MIC/MMC results, at different levels and with sodium phytate as the chelating agent. Preliminary studies on unpreserved formulas were first conducted to assure they supported microbial growth, and also to assess the effectiveness of the neutralizing medium for inoculum recovery. Challenge tests were performed using three different formulation types (see Formulas 1, Formula 2 and Formula 3): an o/w emulsion, a shampoo and a tonic lotion, into which blend 1 was incorporated at various concentrations (see Table 2). For comparison, the same formulations were also tested without preservative systems.
In all formulations, blend 1 was added at the end of the production process at a temperature below 50°C with moderate stirring. The addition of the blend did not significantly impact the viscosity of the emulsion or the transparency of the tonic lotion and shampoo (data not shown). After its incorporation into cosmetic products, the pH value was adjusted with lactic acid, 80%.
Results and Discussion
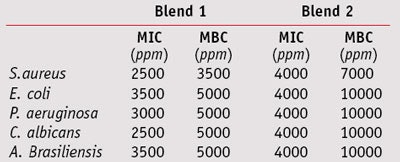
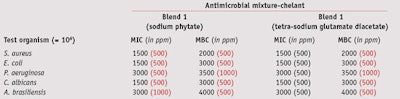
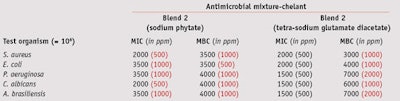
MIC/MCB: To compare the MIC and MBC values of the two blends, their effective concentrations against test microorganisms were determined at a maximum 1% and minimum 0.075% concentration—i.e., the antimicrobial range of activity for these blends. Blend 1 exhibited full bactericidal activity against Gram-positive and Gram-negative bacteria, and fungicidal effects at a concentration of 0.5%. Blend 2 was required at a higher 1% concentration to inhibit both bacteria and fungi (see Table 3).
The antimicrobial activity was also determined in combination with the complexing agents sodium phytate and tetrasodium glutamate diacetate. Notably, their addition caused a slight reduction of viscosity in the emulsion but did not affect product stability or result in separation (data not shown). No such effects were observed in the tonic lotion or shampoo. Results demonstrated that both chelating agents enhanced the antimicrobial effect of the blend tested.
When combined with 0.05% sodium phytate and tetra-sodium glutamate diacetate, blend 1 exhibited a bactericidal action against Gram-positive bacteria at 0.2%, and a complete fungicidal activity against yeasts and molds at 0.3% and 0.4%, respectively (see Table 4). Blend 2, combined with 0.1% sodium phytate, exhibited full bactericidal and fungicidal activity at 0.4%, whereas in combination with 0.2% tetra-sodium glutamate diacetate, it was required at a concentration of 0.7% (see Table 5).
Thus, chelating agents were useful as preservation boosters, although devoid of any biocidal properties, and both improved the effectiveness of preservatives and reduced the amount needed to preserve the formulation. This boosting ability was proven on Gram-negative and Gram-positive bacteria and mold in both blends tested. Noticeably, blend 2 in combination with sodium phytate produced an even better effect than it did with tetrasodium glutamate diacetate.
Challenge tests: After evaluating the antimicrobial activity of the blends, further investigation was conducted to exploit their preservative activity in cosmetic formulations with high water content. Challenge tests were performed using blend 1 at various concentrations in three different formulations, with and without chelating agents. In order to ensure the suitability of the results obtained in the challenge test, the formulations containing the same components were prepared without blend 1, proceeding to the same analysis and artificial contamination carried out for the preserved formulations.
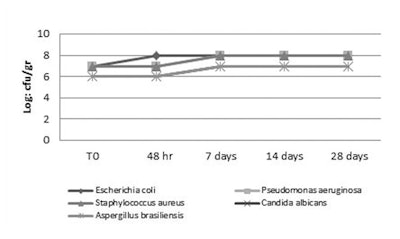
All unpreserved formulations were susceptible to bacterial and fungal contamination, and supported a high level of viable microorganisms during the test period (see Figures 1, Figure 2 and Figure 3). Blend 1 showed effective action, satisfying both the PCPC and European Pharmacopoeia criteria for preservation. A particularly good result was seen in the test with the tonic lotion, see Figures 4, 5, 6, 7 and 8, where all five test microorganisms were completely inactivated in 48 hr at all concentrations tested, with and without chelating agents.
When evaluating the results from the o/w emulsion, blend 1 at 1% was sufficient to protect the formulation against contamination, inactivating P. aeruginosa, S. aureus, C. albicans in 48 hr, and E. coli (2.4 x 103) and A. brasiliensis (1.0 x 104) in 7 days without chelating agents (see Figure 9). Use of the blend at 1.2% in the o/w emulsion also inactivated E. coli in 48 days (see Figure 10) and at 1.4% blend, all test organisms were inactivated after 48 hr (see Figure 11). Interestingly, with 0.1% sodium phytate, the inactivation at 48 hr was observed with 1% of blend 1 (see Figure 12), showing how the booster reduced the concentration required to reach the same antimicrobial effect. In the case of shampoo, 0.8% and 1% blend inactivated the test organisms in 48 hr, except E. coli (1.0 x 102 CFU/g) and P. aeruginosa (1.0 x 104), which were inactivated at 7 days (see Figures 13 and 14). However, all five organisms were inactivated at < 10 CFU in 48 hr with 1.2% blend 1 (see Figure 15). Also observed when analyzing the shampoo results was the fact that the addition of 0.1% sodium phytate significantly increased activity, giving a higher log reduction, in particular of P. aeruginosa and E. coli (see Figures 16 and 17). Thus, this formulation with 0.8% blend 1 also passed the test for broad spectrum preservation against bacteria and fungi according to the PCPC and European Pharmacopoeia.
Conclusions
Several preservatives currently used in cosmetics have been under scrutiny due to safety concerns. Therefore, the need has risen for alternative preservation strategies with effective and non-controversial antimicrobial blends. In the presented study, the authors compared two different mixtures for their antimicrobial activity by MIC/MBC evaluation. Both the mixtures served as effective preservative systems, but blend 1, based on phenethyl alcohol and caprylyl glycol, exhibited particularly high antimicrobial activity.
This blend was then investigated by challenge tests to further explore its range of applications and efficacy in typical cosmetic formulations. It proved to be a well-balanced combination, exhibiting broad-spectrum antimicrobial activity against bacteria, yeasts and molds. The results obtained show that blend 1 provided excellent protection to cosmetics such as water-based products, surfactant based solutions and emulsions, at levels of 0.8-1.2%—largely exceeding the PCPC and European Pharmacopoeia requirements for antimicrobial preservation in cosmetics and topical pharmaceuticals.
The authors also have demonstrated that chelating agents can boost the effects of blend 1, allowing a reduction in the amount needed to achieve the required effect. These results, together with more than five safe years of use in cosmetics, confirm that the blend of phenethyl alcohol and caprylyl glycol can represent a useful and effective alternative to classical preservative systems. This study proves that reliable broad-spectrum preservation without conventional preservatives, and with equal or better performance at the lower use levels, is possible.
References
Send e-mail to [email protected].
1. G Sacchetti et al, Composition and functional properties of the essential oil of Amazonian basil, Ocimum micranthum wild., labiatae in comparison with commercial essential oils, J Agric Food Chem 52, 3486-3491 (2004)
2. K Weber, New alternatives to paraben-based preservative blends, Cosm & Toil 120(1) 57-62 (2005)
3. S Fraud, EK Rees, E Mahenthiralingam, AD Russel and JY Maillard, Aromatic alcohols and their effect on Gram-negative bacteria, cocci and mycobacteria, J Antimicrob Chemother 51(6) 1435-6 (2003)
4. S Silver and L Wendt, Mechanism of action of phenethyl alcohol: Breakdown of the cellular permeability barrier, J Bacteriology 93(2) 560-566 (1967)
5. F Ibarra, J Janichem and W Petersen, Efficacies of different 1,2-alkandiols as antimicrobial agents, SÖFW 134(4) 40-48 (2008)
6. G Bergsson, In vitro inactivation of Chlamidia trachomatis by fatty acids and monoglycerides, in Antimicrobial Agents and Chemotherapy, American Society for Microbiology, Washington, DC USA 42(9) (1998) pp 2290-2294
7. DS Orth, Preservative-free (self-preserving) products, ch 4, in Insights into Cosmetic Microbiology, Allured Business Media, Carol Stream, IL USA (2010) p154
8. SB Levy, AM Dulichan and M Helman, Safety of a preservative system containing 1,2-hexanediol and caprylyl glycol, Cutan Ocul Toxicol 28 (1) 23-4 (2009)
9. IF Burgess, PN Less, K Kay, R Jones and ER Brunton, 1,2-Octanediol, a novel surfactant, for treating head louse infestation: Identification of activity, formulation and randomized, controlled trials, PLoS One 7(4) e35419 (2012)
10. C Oviedo and J Rodríguez, EDTA: The chelating agent under environmental scrutiny, Quim Nova 26(6) 901-905 (2003)
11. JJ Kabara and DS Orth, Preservative-free and self-preserving cosmetics and drugs, ch 9 in Chelating Agents as Preservative Potentiators, Marcel Dekker, NY (1996) p 220
12. RM Richards and RJ McBride, The preservation of ophthalmic solutions with antibacterial combinations, J Pharm Pharmacol 24 (2) 145-148 (1972)
13. W Siegert, Can new biodegradable complexing agents replace tetrasodium EDTA to boost preservatives? SÖFW 1-2 (2008)
14. B Brewster, Preservative boosters: Up against the wall, Cosm & Toil 122(3) 26-34 (2007)