Industry expert Tony O'Lenick explains the difference between a betaine and an amphoteric...
Amphoteric surfactants are a class of compounds that have both a positive (+) and negative (-) charge in the same molecule. The amphoteric class of compounds has, within it, several additional types of compounds, depending on how the molecule’s charge changes as a function of pH. A betaine is simply one type of amphoteric.
Betaines
One class of compounds within the amphoteric family is the betaine. Because of its structure, it can exist in only two ionic forms, zwitterionic and cationic. Betaines have the following structure:
The structure shown is a zwitterionic form, having both positive and negative charges present in the molecule. As the pH is lowered, the carboxyl group is protonated and a compound with a positive (+) charge develops. This structure is:
This explains why betaines provide better conditioning in low-pH systems. Since the nitrogen is surrounded by carbon atoms, it cannot lose its positive (+) charge by pH alteration.
Ampholytes
In contrast to betaines, ampholytes are compounds that can exist in three different forms: anionic, cationic and zwitterionic, depending on the pH level. The structures shown below are arranged from low pH to high pH.
Cationic form (carboxyl group and amino group protonated):
Zwitterionic form (can be insoluble in water):
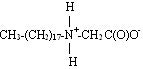
Anionic form:
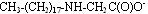
These materials are used in a variety of formulations and rarely are used alone. The structure that exists at a given pH will result in interactions with other ingredients, resulting in higher viscosity, improved conditioning, improved foam or better wet combing. In fact, by properly managing and optimizing these interactions, formulators can adjust formulations and optimize them to work more effectively.










