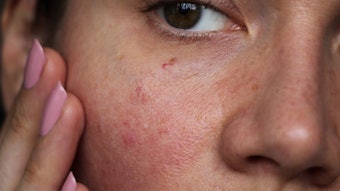
SkinMedica has announced the FDA approval of a new treatment for mild to moderate atopic dermatitis. The company announced that the U.S. Food and Drug Administration (FDA) approved its new drug application for Desonate (desonide gel).
The product, according to the company is a low potency topical steroid formulated in a proprietary water-based hydrogel vehicle developed and patented by Dow Pharmaceutical Sciences Inc. The formula reportedly is free of alcohol, fragrance and surfactants, which can irritate or dry sensitive skin. The product was created as a result of the joint effort between SkinMedica and Dow. It will be available to physicians in early 2007.